May_New Drugs|GLP-1 weight-loss drugs: second wave of gold rush
GLP-1New weight-loss drugs are popular all over the world, and the shortage of goods and production capacity continues to burn.
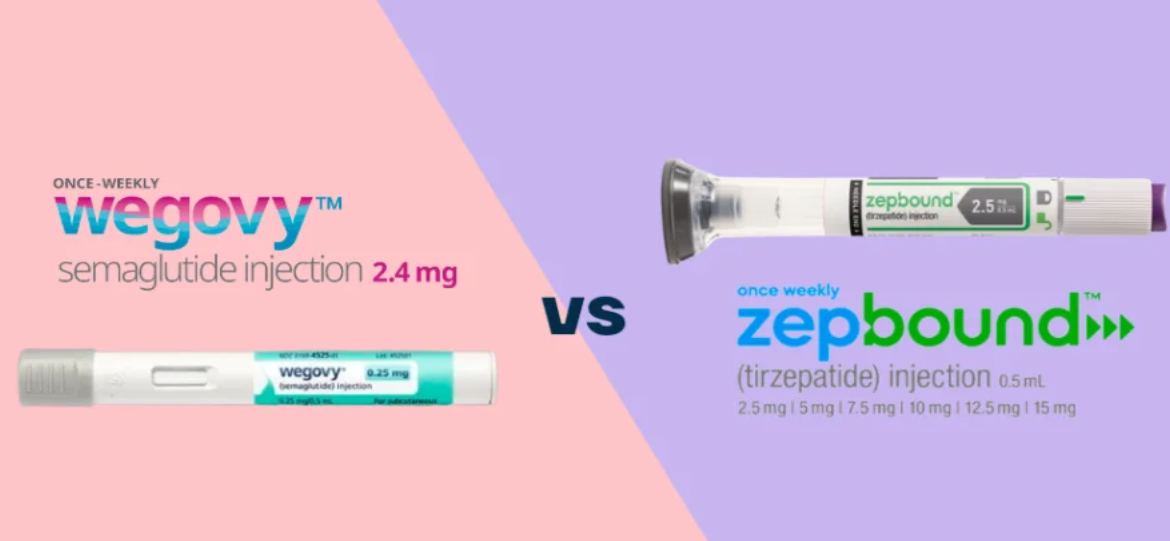
How popular are the new GLP-1 weight loss drugs? At present, the only two new GLP-1 weight loss drugs approved for marketing in the world are Semaglutide (trade name Wegovy), which will be approved by the U.S. FDA in June 2021 by Novo Nordisk.®), and Eli Lilly's November 2023 U.S. FDA approval of Tirzepatide (trade name Zepbound®). Judging from the recent strong sales figures of the two pharmaceutical companies, Lilly Zepbound®175 million in revenue in less than two months since IPO, and Novo Nordisk's 2023 Wegovy®Under the premise of insufficient production capacity and focusing on the market in 8 countries, the company has sold US$4.5 billion, which is a good result if the sales of two other drugs with the same ingredient, Semaglutide (trade name Ozempic), are taken into account.®and Rybelsus®In the past, Novo Nordisk's Semaglutide was approved for hypoglycemia, and may be used off-label. Novo Nordisk's Semaglutide ingredient alone generated about US$21.2 billion, while Eli Lilly's Tirzepatide also had a production value of about US$5.3 billion. Under the smoke and mirrors of tight supply and competition for market share, Novo Nordisk and Eli Lilly are coincidentally expanding their production capacity with large sums of money. Novo Nordisk has acquired the OEM drugmaker Catalent with US$16.5 billion and invested in the expansion of its plant in Denmark with US$6 billion, while Eli Lilly has spent US$2.5 billion on the construction of a new plant in Germany and US$1.6 billion on expansion of its plant in the U.S. It is easy to find out that there is a huge opportunity for the new drug GLP-1 to reduce the weight of the population under this circumstance. In view of this, GLP-1 weight loss drug is also regarded as an opportunity for competition to seize the market.
Remarks: Off-label use: This refers to the use of a medication prescribed by a physician that is not in accordance with the indications in the generic version of the medication.