March_ New Drugs|New Market Focus: CAR-T Cell Drugs
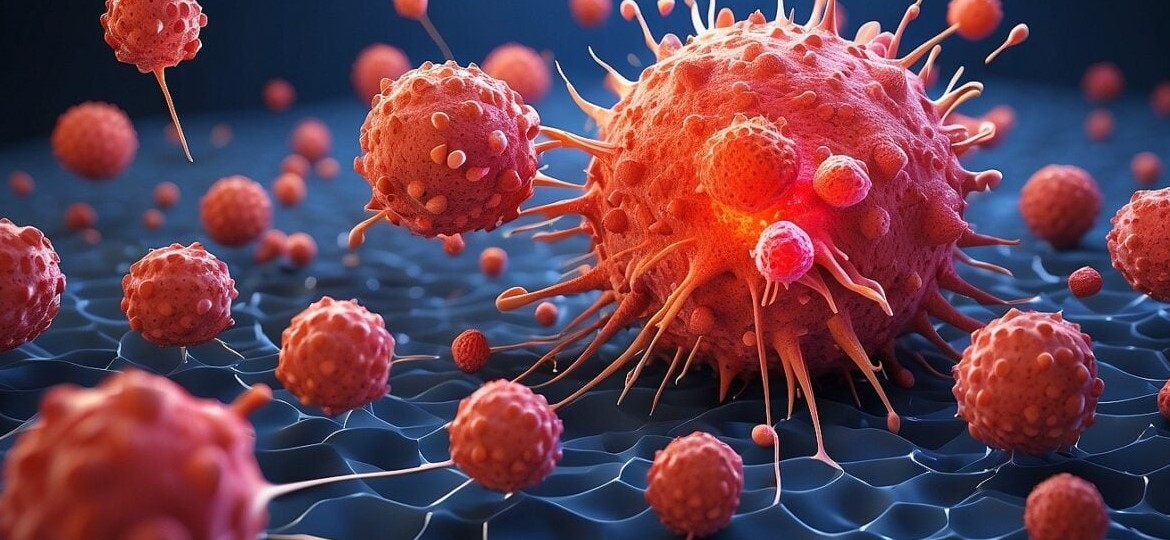
CAR-T cell new drug (Chimeric antigen receptor T cell, chimeric antigen receptor T cell) has been approved by the U.S. FDA for the treatment of blood cancer, lymphoma, and multiple myeloma, etc., especially for patients who have already tried many kinds of treatments (chemotherapy and stem cell transplantation) and failed to achieve the effect of patients can be completely cured by CAR-T 療法, which has aroused great concern! Separate T-cells (a kind of human autoimmune cells with offensive power) from the patient's blood, and embed special gene fragments through genetic engineering, so that the T-cells will have the ability to identify tumors, i.e., installing a CAR detection navigation system on the T-cells (just like armed soldiers) that can track cancer cells (such as a navigation helmet for detecting the enemy), and then massively expanding the CAR-T immune army that can eliminate cancer cells, so that the cancer cure rate can reach approximately 1,000 percent with precise elimination. The success rate of cancer treatment is more than 50%, but there is still a possibility of recurrence or side effects such as cytokine storm, neurotoxicity and abnormal activation of white blood cells. Compared with other cancer treatment technologies, CAR-T cell drugs use living cells as a weapon to attack blood cancers, and clinical studies have proved that the cells of some patients can still survive in their bodies after more than 10 years of administering CAR-T cell drugs, therefore CAR-T cell drugs are regarded as the most effective cancer treatment technology. Therefore, CAR-T cell drugs are considered to be able to achieve long-term cancer control and cure.