Jan W4|Big Health News Highlight: Ropeg's New Indications Recognized by NCCN, Yoho Pharmaceuticals ET Takes Another Step Toward the U.S. Market
Jan W4|Big Health News Highlight: Ropeg's New Indications Recognized by NCCN, Yoho Pharmaceuticals ET Takes Another Step Toward the U.S. Market
PharmaChem announced that its new drug Ropeg has been included in the latest National Comprehensive Cancer Network (NCCN) guidelines for the new indication of Essential Thrombocythemia (ET) and has been categorized as Category 1 for "patients with high-risk ET who have not responded favorably to or failed to respond to current therapies". Preferred.
Ji-Pu Viewpoint:
After the approval of a new drug, revenue challenges mainly come from the subsequent marketing and commercialization process, which includes market penetration and physicians' willingness to adopt, the impact of health insurance and payment systems on the actual utilization rate of patients, competition from other competing drugs or alternative therapies, as well as differences in local drug prices, regulations, and payment systems. Ropeg (trade name: BESREMI), the new drug of Pharmaceuticals China, has been gradually stabilizing the market for PV (polycythemia vera). Ropeg (trade name BESREMI) has gradually gained a firm foothold in the PV market. With the extension of the second new indication, Essential Thrombocytosis (ET), progressing towards the FDA approval stage in the U.S. (expected to be approved by August 30th, 2026), and the third indication, Pre-fibrotic Primary Bone Marrow Fibrosis (PBMBF), it has the potential to be expanded to the overall myeloproliferative neoplasms (MPNs), which are the most common forms of PBMBF. Neoplasms (MPNs) include chronic myelogenous leukemia (CML), true erythrocytosis (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). In addition, there are rare types of hematopoietic stem cell-derived blood cancers such as chronic eosinophilic leukemia (CEL), chronic neutrophilic leukemia (CNL), and juvenile myelomonocytic leukemia (JMML). In addition, the inclusion of the NCCN guidelines for PV and ET medication will accelerate insurance companies' coverage decisions, which will lead to significant revenue growth in the future.
Related news link: News
Dec W2|Big Health News Highlight: Renishin Steger's disease phase III trial successfully clears blindness, to be reviewed by U.S. FDA next year
Belite, a subsidiary of Jen Sun Pharmaceutical, announced that it has successfully unblinded its Phase III clinical trial (DRAGON) of LBS-008 (Tinlarebant), a new oral drug targeting juvenile Steger's lesion, and is planning to submit an application for registration of a New Drug Inquiry (NDA) to the U.S. FDA in the first half of 2026.
Ji-Pu Viewpoint:
Stargardt disease is a hereditary eye disease (a common genetic retinal disease) that gradually takes away vision and may eventually lead to blindness, for which there is currently no approved treatment worldwide. Belite Bio, a subsidiary of Jen Sun Pharmaceuticals, announced that its new oral drug LBS-008 (Tinlarebant) for juvenile Steger's disease has been successfully unblinded in its Phase 3 clinical trial (DRAGON), with a 5mg/day dosage resulting in an average reduction of approximately 80% in RBP4 levels from baseline, and a significant reduction in the rate of increase in retinopathy compared to the placebo group. 36%, Belite Bio plans to discuss the potential follow-on program for Tinlarebant and the submission of the Investigational New Drug (IND) registration application with regulatory authorities in each country in the first half of 2026, and submit the IND registration application to the U.S. FDA.
Steger's disease is a genetic defect that alters the way the body processes vitamin A, which is used to make retinal cells. The defect causes yellow clumps to form in the eye, damaging cells and causing progressive vision loss. The value of this case lies in the fact that Tinlarebant, a retinol binding protein 4 (RBP4) antagonist, is a First-in-Class Drug designed to slow or inhibit the progression of Steger's Disease (STGD1) to prevent the accumulation of the vitamin A toxin (ditretinoin) that causes STGD1 and contributes to the mechanism of the development of dry AMD. Tinlarebant works by lowering serum levels of retinol-binding protein 4 (RBP4), the carrier protein that transports retinol to the eye. By regulating the amount of retinol entering the eye, Tinlarebant reduces the formation of bivalvia to protect the health of retinal tissue. The pathophysiology of dry macular degeneration (dry AMD) is similar to that of Steger's disease (STGD1), in that the over-accumulation of toxic bisretinoids in the retina causes photoreceptor cells to die, resulting in blindness.
Therefore, the clinical phase 3 approval of Tinlarebant demonstrates the actual advantages of its safety and efficacy, and it is also expected to challenge the dry macular degeneration with extended indications, expanding the market for the indication group. Currently, Belite Bio has already completed a US$350 million increase. According to U.S. investment bank estimates, about 50,000~53,000 people in the U.S. suffer from Steger's disease, which is currently a drug-free market, and based on relatively conservative drug price estimates, the market size for Steger's disease (STGD1) will be around US$1.27 billion by 2030, and analysts from the investment bank Leerink Partners predict that Analysts at Leerink Partners Investment Bank predict that annual sales of the drug will peak at US$1.4 to US$3.6 billion. In addition, potential competitors in the market include Alkeus Pharmaceuticals, or gene therapy developers such as Ocugen, AAVantgarde, and Splice Bio.
New drug certification is the first step in the commercialization of a drug, and the real value of a drug is still to be verified by its actual sales in the pharmaceutical market after its launch. For rare disease drugs, market promotion relies on the support of government policies and philanthropic funds. According to a study conducted by SDC Thomson Reuters and S&P Capital IQ Commercial Database, the rate of return on investment for orphan drugs for rare diseases is generally better than that for non-orphan drugs, indicating that this area not only has clinical and social significance, but also shows relatively outstanding commercial potential.
Related news link: News
Sep W1|Big Health News Highlight: U.S. sees 'biggest health care hike in nearly 15 years'! Insurance giants say Trump's tariffs are a disaster, and tens of millions of people are facing a double whammy.
At a time when U.S. families are already feeling the pinch from high prices, the U.S. public is facing the biggest increase in health insurance premiums in 15 years, according to the Financial Times. UnitedHealth, one of the giants of the insurance industry, blamed the increase on the "high level of uncertainty" brought about by Donald Trump's tariff war.
Ji-Pu Viewpoint:
At the beginning of the year, Trump adopted a series of policies and programs to reduce the burden of healthcare in the U.S. However, in reality, U.S. healthcare expenditures are still rising, and the prices of medical products and the utilization of healthcare services have both climbed, reflecting the intensifying pressure on U.S. healthcare insurance costs.
Both the government-backed Medicare, Medicaid and Obamacare programs, as well as private insurers UnitedHealth Group (UNH-US), Centene (CNC-US) and Molina Healthcare (MOH-US), and Elevance Health (ELV-US), continue to face upward pressure on healthcare costs. UNH-US, Centene (CNC-US) and Molina Healthcare (MOH-US), as well as Elevance Health (ELV-US) continue to face upward pressure on healthcare costs due to factors such as the introduction of expensive new drugs (e.g., weight-loss medications) and cancer therapies into the marketplace, the increased bargaining power of large, consolidated healthcare systems, which has led to higher prices for services, as well as inflation that has pushed up the cost of healthcare payrolls. In anticipation of continued high healthcare costs, the end of Obamacare subsidies at the end of 2025, significant premium increases, tightening of related health insurance or subsidy standards, and reductions in healthcare benefits (elimination of premium subsidies), with the cost of insurance being passed on to businesses or employees, the U.S. appears to be on a gradual path toward healthcare austerity.
Related news link: News
Aug W1|Big Health News Highlight: Trump winds down the ball, long term drug supply chain restructuring feared
The U.S. announced the reciprocal tariff rate of 20% on 8/1, which is higher than Japan and Korea's 15% and slightly lower than India's 25%. Although the current project does not include pharmaceuticals, U.S. President Donald Trump has recently issued a letter to a number of international pharmaceutical companies requesting that they provide U.S. "Most Favored Nation (MFN) drug prices," which may still have an impact on the Taiwan pharmaceutical market.
Ji-Pu Viewpoint:
Pharmaceuticals are not affected by the tariffs for the time being, but the duty-free treatment they have historically enjoyed may be loosened! A major change in U.S. policy regarding tariffs on pharmaceuticals will break the long-standing practice of exempting pharmaceutical imports from duty. According to the latest U.S.-European Union Trade Agreement and Reuters and Irish News, the U.S. will impose a 15% tariff on drugs from the European Union, although some generics may be exempted, the scope of which has not yet been clarified. The tariff could significantly drive up costs as the EU supplies about 60% of imported drugs to the US, particularly 43% of branded pharmaceutical ingredients (APIs). The move comes at a time when U.S. President Donald Trump has offered a 60-day price reduction order, pressuring pharmaceutical companies to lower drug prices within the United States. On the one hand, he is raising the cost of importing drugs externally, and on the other hand, he is demanding that pharmaceutical companies lower their prices. In the long run, the supply chain may shift to the U.S. and push up the cost of drugs for international pharmaceutical companies.
Related news link: News
Trump drug tariffs finally realized in new U.S.-EU trade deal |fiercepharma|Fiercepharma
Jul W1|Big Health News Highlight: Trump's 'Bigger is Better Act' passes! Congress passes largest tax cut bill in history: US national debt could soar by $3.4 trillion, health insurance for millions in jeopardy.
According to an analysis by the Congressional Budget Office (CBO), the bill would cut government spending by $1.1 trillion over the next decade, much of which would come from Medicaid. The program is a critical safety net that provides health care coverage to more than 71 million low-income people across the United States. The bill would tighten application standards and impose work requirements that are expected to result in nearly 12 million people losing their eligibility for health care coverage.
Ji-Pu Viewpoint:
The Great America Act: Senate cuts to Medicaid will result in a significant increase in the number of uninsured in the U.S. Medicaid payment reform weakens states' authority to raise subsidies, reducing hospital revenue sharing from 61 TP3T to 3.51 TP3T in the 40 states that have already expanded health care coverage. Some people will need to work in order to continue to receive Medicaid; 2. Funding will be reduced for health care services in rural communities in the U.S. may be reduced; 3. ACA coverage (Obamacare) will be more difficult to obtain; 4. Medicaid beneficiaries will have to pay more for office visits; 5. Some immigrants will lose access to the subsidized ACA program; and in general, the U.S. is rectifying the chaotic situation of health care resource wastage by reducing health care expenditures, which has led to a tightening of the abundant U.S. medical resources. Overall, the U.S. is rectifying the wastefulness of its health care resources and cutting back on health care expenditures.
Related news link: News
Jun W1|Big Health News Highlight: Buying of epidemic prevention products increases Tabor and Bowling rush to produce rapid screening reagents
The new crown of the epidemic for seven consecutive weeks to warm up, coupled with the Dragon Boat Festival holiday, fear of increasing the risk of transmission of infection, the domestic chain of drugstores industry, fast screening reagents are in a state of shortage, cleaning and disinfecting supplies and prevention of epidemic-related medicines part, has appeared to be higher than the weekdays of 10% to 20% of the buying gas, the new crown of the fast sieve manufacturer Bao Ling Fu Jin, said that it is expected to create diagnostic department of the double-digit growth of revenues.
Ji-Pu Viewpoint:
The new crown epidemic is heating up, and the CDC of the Ministry of Health and Welfare has warned that this year's new crown epidemic is likely to peak at the end of June and the beginning of July, and the weekly number of new cases is likely to reach 200,000 by then. The new crown epidemic has caused the market to worry mainly about the hundreds of cases of severe illness and 19 deaths, and against this background, the business opportunities for epidemic prevention have quietly arrived, and the epidemic prevention products such as quick-screening reagents, alcohol, masks, and wet wipes are becoming highly sought after commodities in the market, which in turn has led to a double-digit growth in revenue for relevant supply chain manufacturers. Related supply chain companies are optimistic about double-digit revenue growth. It is too early to say whether the new outbreak will return, but observing whether the number of confirmed cases will continue to surge or double will be an important indicator for the future.
Related news link: News
May W1|Big Health News Highlights: Trump's tax effects lead international medtech firms' conference
Johnson & Johnson, Abbott and Boston Scientific have all said the White House's tariff policy will cost them hundreds of millions of dollars, but annual forecast growth remains unchanged or even increases.
Ji-Pu Viewpoint:
The U.S. announced on April 2 that reciprocal tariffs imposed on the rest of the world would return to the standard rates from July 9, the same as before the suspension. Tariffs on medical devices and equipment are inevitable, and they are a major concern for medical technology companies this year. A number of medical device and life science equipment manufacturers, including Stryker, GE Healthcare, Johnson & Johnson, Abbott, Boston Scientific, and Danaher, have predicted that the cost of tariffs will cost their companies hundreds of millions of dollars, impacting profitability. As a result, international medical technology companies have formulated short- and long-term response plans, such as surcharges, supply chain management, cost actions, and shifting manufacturing to change manufacturing footprints, adjust supply chains, increase surcharges, and cut costs to limit the impact of the tariffs, which are expected to be realized in the Q3 financial reports. Enterprises with globalized production capacity and technological innovation will be more resistant to policy shocks and geopolitical risks. In addition, medical technology companies will shift from scale expansion to value restructuring as the main axis of development.
Related news link: News
Apr W4|Big Health News Highlight: Pitchbook Warns of Reduced M&A Activity and Venture Capital in Biopharmaceutical Industry
Pitchbook says the biotech industry is shifting to a capital-efficient, US domestic market-centric model. In this protectionist environment, AI consolidation and manufacturing innovation will determine market winners, and as tariffs force manufacturing back to the U.S., Pitchbook warns of a decline in M&A activity and VC funding.
Ji-Pu Viewpoint:
The Trump drug tariffs threaten to further depress the stock prices of large pharmaceutical and biotechnology companies as the U.S. Department of Health and Human Services (HHS), the FDA, the CDC and the NIH lay off many of their executives and personnel, destabilizing the U.S. biopharmaceutical market and increasing the volatility of the U.S. capital markets. In addition to creating financing challenges for new biotechnology start-ups in the capital markets, the tariffs are expected to force international pharmaceutical companies to choose sides, potentially adding significant financial costs to manufacturers seeking to establish new manufacturing capacity in the U.S., and potentially cutting into the number of large, cash rich international pharmaceutical companies that may need to invest in their future projects. In addition to creating financing challenges for new biotechnology companies in the capital markets, the tariffs are causing cash-rich large international pharmaceutical companies to reexamine their future investments. It is anticipated that the tariffs will force large international pharmaceutical companies to choose sides, which may result in significant additional financial costs for pharmaceutical companies seeking to establish new manufacturing capacity in the U.S., which may result in reduced R&D expenditures or mergers and acquisitions, and may result in a reduction in venture capital funding for venture capital firms as a result of the impact of the loss of the capital market, which would result in financing constraints for the U.S. biotechnology industry.
Related news link: News
Apr W2|Big Health News Highlight: Trump's reciprocal tariffs on hold for 90 days! The latest global tax rates at once!
U.S. President Donald Trump announced that tariffs on 75 countries around the world will be reduced to 10% for 90 days as an observation period for the negotiations, but tariffs on Chinese imports will be raised significantly to 1,25% with immediate effect.
Ji-Pu Viewpoint:
The U.S. once again wielded the tariff stick, and on 4/2 Trump signed the "reciprocal tariff" executive order, imposing a "universal" tariff of 10% on more than 180 countries around the world, which is based on the impact of non-tariff barriers and currency manipulation in each country to set a comprehensive tax rate. 57 countries and regions with large trade deficits with the U.S. are subjected to a higher reciprocal tariff on top of the 10%, although categories such as iron and steel, aluminum, and copper have survived this tariff impact for the time being, and on 4/10 the tariff policy has made a temporary and temporary U-turn, Although steel, aluminum, copper, semiconductors, and pharmaceuticals have been spared from the impact of this tariff, and the 4/10 tariff policy has made a sharp turnaround for the time being, the U.S. government is still in the process of implementing the 4/10 tariff policy.
However, the tariffs have plunged the biotech and medical industries into "uncharted territory". Medical devices and equipment have been included in the scope of tariff increase and are directly affected; pharmaceuticals (finished drugs) and APIs listed on the front side of the Annex to the Executive Order have been spared, but Trump has openly stated that he will not rule out the imposition of 25% tariffs on drugs, creating a high degree of policy uncertainty, which has caused biotech-medical industry players around the world to continue to pay close attention to Trump's future policy direction. In addition, the possibility of rapid policy changes and ever-changing tariff strategies make it difficult for the biotech and medical industry to make major supply chain shift decisions in the short term.
Related news link: News
Mar W4|Big Health News Highlight: After Trump Threatens Tariffs, Jasmin Joins Pharmaceutical Peers in $55 Billion Stimulus to U.S. Manufacturing Industry
After President Trump threatened to impose high tariffs on drugs, Jiao Sheng followed suit and invested billions of dollars in U.S. manufacturing. Pfizer made a similar commitment.
Ji-Pu Viewpoint:
With Trump urging the manufacturing industry to return to the United States, as well as hinting at a 25% tariff on U.S. drugs, the U.S. pharmaceutical industry has begun to take a stance on moving back to the U.S. The trend has been formed, and there have already been major announcements by Eli Lilly, Merck Sharp & Dohme (MSD), and Amgen, and Jiao Sheng (John & John), which have emphasized their investment in the U.S. manufacturing industry. Additionally, AstraZeneca, a major British company, announced a $2.5 billion investment in a global strategic R&D center in Beijing, China.
In recent years, China's rapid rise in biopharmaceutical innovation capacity, is from the global pharmaceutical OEM factory, transformed into a drug R & D innovation center, China and the United States in the biotechnology and pharmaceutical industry competition will be from the biosafety law to take measures to compete, all the way to the U.S. biotechnology and pharmaceutical industry back to the United States and tariffs and trade wars, the competition in the future will not only be confined to the market competition, but also involves the breakthroughs in new technologies, medical data security, and the supply chain layout and so on. The future competition will not only be limited to market competition, but also involve new technology breakthroughs, medical data security, and supply chain layout.
Related news link: News
Mar W2|Big Health News Highlight: Trump Signs Executive Order Urging Transparency in Health Care Pricing
The New York Times reports that President Trump signed an executive order on July 25 that renews a push for one of his favorite health care policies in his first term: public transparency on health care prices. The new executive order will assign the Department of Health and Human Services (HHS), the Treasury Department, and the Department of Labor to consider more effective ways to help patients learn about health care prices.
Ji-Pu Viewpoint:
Under the Trump administration, changes are unfolding in the U.S. pharmaceutical industry. From the inauguration to the present, from the Department of Governmental Efficiency (DOGE)'s review of Medicare and Centers for Medicare & Medicaid Services (CMS) wasteful expenditures, to the wave of layoffs at the CDC, NIH, and FDA, which are the pillars of the U.S. public health, and the drastic cuts in scientific research and freezing of the budgets for pharmaceutical research and development, and to the increase in the transparency of the prices of healthcare services, which is an attempt to make the prices of healthcare services openly known only in the private negotiation among doctors, hospitals, pharmaceutical companies, and insurers. Under this change, it is expected that the review of new drugs and innovative medical materials will gradually enter a tortoise-speed era, and the trade barriers between the U.S. and China and the austerity environment in medicine will lead to a new change in the healthcare supply chain in the largest single healthcare-demanding market in the U.S., with a population of 330 million people. The U.S. trade barriers and pharmaceutical austerity will lead to new changes in the healthcare supply chain in the largest single healthcare market in the U.S. with a population of 330 million.
Related news link: News
FEB W2|Big Health News Highlights: Mask Government Efficiency Gets Access to CMS
According to the Wall Street Journal, Department of Government Efficiency (DOGE) officials are focusing on possible fraud or wasteful spending within the Centers for Medicare and Medicaid Services (CMS), which provides health insurance to more than 160 million Americans through Medicare, Medicaid, the Children's Health Insurance Program, and the Affordable Care Act, and whose expenditures in FY 2024 amount to about $1.5 trillion. Its expenditures reached about $1.5 trillion in FY2024, accounting for 22% of the federal total.
Ji-Pu Viewpoint:
With the new Trump administration, the U.S. healthcare program is facing turmoil as the Department of Government Efficiency (DOGE) begins a review of the Centers for Medicare and Medicaid Services (CMS) and freezes large amounts of federal spending. In addition to temporarily prohibiting states from using Medicaid payments, tariffs and a broader trade war have potential implications for the future supply of U.S. drugs and medical equipment.
Medicare and the Centers for Medicare & Medicaid Services (CMS) are in charge of U.S. Medicare and Medicaid payments, while intermediary Pharmacy Benefit Managers (PBMs) are responsible for brokering drug price negotiations with pharmaceutical manufacturers, and CMS or commercial health insurance organizations, and conducting health insurance settlements, along with the U.S. efforts to rectify the chaotic situation of healthcare resource wastage and reduce the trend of healthcare spending. With the U.S. trend of rectifying healthcare waste and reducing healthcare expenditures, the overall scale of pharmaceutical expenditures is bound to be affected in the future.
The U.S. healthcare system relies heavily on international sources for drugs (e.g., anti-cancer drugs) and medical equipment, and China is the source of disposable and small products such as needles, syringes, stethoscope covers to protect patients from infection, gowns, gloves, masks, respirators and other protective equipment, pulse oximetry, anesthesia equipment, and blood pressure monitors, which are commonly used in many hospitals, and an increase in tariffs could drive up the cost of products along the supply chain. An increase in tariffs would increase costs in the supply chain, which may inadvertently jeopardize the supply of products and is a cause for ongoing concern as to whether it will accelerate the replacement of the new supply chain.
Related news link: News
Mask Department of Governmental Efficiency Receives CMS Access Rights|healthcaredive
Jan W3|Big Health News Highlights: 5 Insights from the JPMorgan Healthcare Conference 2025
Forbes reported that the J.P. Morgan Healthcare Conference 2025 described five key trends in biopharmaceuticals, including increased deal activity, the looming patent cliff, the search for new R&D projects, and the trend in deal size that will continue; health policy issues, and the uncertainty surrounding the future of Trump's new health policy; the rise of Chinese innovation, and the rising influence of Chinese innovation in the biopharmaceutical space; and the dominance of AI in pharmaceuticals, with a clear consensus that Chinese innovation is on the rise. uncertainty; the rise of Chinese innovation, the rising influence of Chinese innovation in the biopharmaceutical field, the dominance of AI in pharmaceuticals, the consensus is clear that AI will revolutionize the way drug development is conducted, as well as advances in cellular and gene therapies, which continue to gain momentum.
Ji-Pu Viewpoint:
J.P. Morgan Healthcare's annual meeting has always been a wind vane for international pharmaceutical companies to influence their strategic layout and the direction of the global pharmaceutical industry. 2025 will feature J&J's US$14.6 billion acquisition of Intra-Cellular Therapies to expand central nervous system drugs; Lily's US$2.5 billion acquisition of Scorpion Therapeutics' PI3Kα inhibitor program to bet on the best-in-class mutant selective PI3Kα inhibitor for breast cancer; or AbbVie's (Abovir) acquisition of Scorpion Therapeutics' PI3Kα inhibitor program. Scorpion Therapeutics' PI3Kα inhibitor program, betting on the best-in-class mutation-selective PI3Kα inhibitor for breast cancer; or Abbvie's $1.05 billion deal with Simcere Zaiming to develop SIM-0500, a humanized GPRCSD-BCMA-CD3 trispecific antibody. antibody SIM-0500 for the treatment of refractory multiple myeloma. With the pressure of patent cliffs and loss of market exclusivity for best-selling drugs, as well as the emergence of new technologies, acquisition of potential assets or advanced technology platforms remains an important strategy for international pharmaceutical companies to maintain long-term growth and competitiveness, with their preferred topics being neuroscience, oncology, and immunology, among other large market areas.
Related news link: News
5 Insights from the 2025 JPMorgan Healthcare Conference|Forbes
Jan W1|Big Health News Highlight: FDA Drug Approvals 2024: Smaller Companies Stand Out in Many Key Approvals
The US FDA New Drug Approval List shows a track record of 68 new drugs (including vaccines and cellular therapies) approved by the US FDA in 2023 according to Fierce Pharma's annual report, with 43 (63%) being developed by or co-participating with companies with sales of up to US$3 billion. Compared to 2024, only 23 of the 55 FDA-approved new drugs (including vaccines and cellular therapies) (42%) were developed or co-developed by companies with $3 billion in sales in 2023, with smaller companies showing a greater willingness to market their products on their own than to bring in large pharmaceutical partners.
Ji-Pu Viewpoint:
In 2024, the U.S. FDA approved a total of 50 new drugs (excluding vaccines and cellular therapies), 32 of which were new small molecule drugs (NCEs) and 18 were large molecule drugs (NBEs), and the total number of new drugs approved for the treatment of rare diseases and cancers led the list of approved new drugs. There are also a number of new drugs approved in the areas of anti-infective and central nervous system diseases. 2024 is different from the past in that there are many small new drug companies such as Ligand, Iovance, Allecra, Hugel, BeiGene, Madrigal, Kyowa Kirin and Idorsia, etc., which are more willing to market their products on their own, and the competition and cooperation in the development of new drugs will continue. In the game of competition and collaboration in new drug development, large pharmaceutical companies are more inclined to acquire late-stage assets (clinical phase 3 candidates); it may not be the best option for small pharmaceutical companies to bring in large pharmaceutical companies for commercial collaboration.
Related news link: News
Dec W4|Big Health News Highlight: Children under 14 worst hit? Is the Human Interstitial Pneumonia Virus (HIPV) spreading to Taiwan?
Since the beginning of winter, the number of human mesopneumonia virus (hMPV) infections in China has increased significantly, and there are even rumors that its fatality rate is as high as 43%, prompting concerns about whether it will become the next "New Crown Epidemic"!
Ji-Pu Viewpoint:
Autumn and winter transition season is the peak season for colds and influenza, which may come from a variety of different influenza viruses, rhinoviruses, plasmodium pneumophila, respiratory fusion viruses, human mesopneumonia virus and other pathogens infected, according to international data, respiratory infectious diseases are showing a continuous trend of increase in the warming trend of the main infectious agent in Mainland China is the human mesopneumonia virus, infected with children under 14 years of age, the epidemic supply chain is mainly the vaccination (masks) community, testing and detection community or more towns. If the epidemic continues to spread to the adult population or more towns in mainland China, we must be more vigilant. The supply chain of the epidemic mainly consists of the vaccination (masks) community, the testing community and the medication community, although there is no vaccine against the spread of Interstitial Pneumonia Virus (IPV) infection or therapeutic drugs for the time being, and it is just a supportive treatment, it is still too early to say whether the gathering of people at the festivals has led to a pandemic, and this has to be monitored in the future.
Related news link: News
Dec W2|Big Health News Highlight: AI medical treatment in the field, Taiwan companies compete in the international cup.
Taiwan Medical Technology Exhibition closed on the 8th, this year AI jumped to the main show, digital medical products, medical management solutions gradually landing, hospitals, technology companies welcome orders and cross-border cooperation case, is expected to help the medical industry to enter the international arena. According to the inventory of the Health Policy Council, this year's medical technology exhibition, reduce the burden on health care, help doctors and patients to communicate, precise surgical treatment, acute and severe disease alert, precise diagnostic screening, AI drug research and development of the six major AI applications of science and technology products has become a trend, attracting the attention of hospitals and other international buyers, and has the opportunity to drive Taiwan's health care industry to start a new momentum.
Ji-Pu Viewpoint:
This year's MedTech Taiwan focuses on the fields of smart hospitals, specialty medicine, digital medicine, smart health, medical equipment, precision testing, biopharmaceuticals, regenerative medicine, preventive medicine, and long-term care for the elderly. With years of resource investment, Taiwan's MedTech medical technology products, in addition to more in line with the actual field of medical application, and improve medical efficiency, through AI-enabled and product iteration and upgrading, and gradually enhance the visibility in the international market, especially the trend of integration and connectivity between various medical and digital health platforms has been gradually formed, and it is expected that in the digital healthcare platform services (Acer, Quanta, Wyndham), products (ASUS, Inventec, Wistron), the company will be able to provide the most advanced medical technology products and services in the world, and will also be able to offer the best medical technology products in the world, It is expected that vendors with a presence in digital healthcare platform services (Acer, Quanta, and Wynn), products (ASUS, IAC, Wistron), and industries (Acer GIS) will gradually magnify their profitability synergies.
Related news link: News
Taiwan's AI medical treatment is in the market|Commercial Times|Taiwanese Medical Journal
Nov W4|Big Health News Highlight: Healthcare reforms expected to improve drug coverage regulations, benefits for national pharmaceutical industry
Key points of the Health Insurance Drug Price Amendment Law: In order to encourage domestic pharmaceutical companies to manufacture innovative drugs, the Health Insurance Administration (HIA) will provide preferential pricing for new drugs that are approved within two years of international marketing authorization, or approved for marketing in the ten most advanced countries for five years, but with a new domestic ingredient, as compared to Taiwan's first-ever new drugs. Secondly, in order to encourage the timely introduction of generic or biosimilar drugs into the market and to provide prescription drug choices, the Department of Health Insurance (DHI) will offer the same maximum price to the first two domestically manufactured generic or biosimilar drugs that have obtained a drug license within five years of the expiration of the original manufacturer's license. Domestically manufactured drugs will be given preferential pricing to stabilize the supply of drugs, including the use of domestically produced APIs, or domestic safety clinical trials published in international academic journals and added to the generic list, as well as the earliest to file a P4 patent claim with the competent authority and obtain a drug license, as long as one of the three conditions is met, the health care insurance will be able to increase the price of any one of the three conditions in the future by 10%.
Ji-Pu Viewpoint:
The Ministry of Health and Welfare encourages local pharmaceutical companies to produce new drugs, biosimilar drugs, and high-quality generic drugs. The policy to increase the use of biosimilar drugs from 7% to 30%, as well as the amendment of the health insurance drug price to favor the drug price payment, will push up the scale of manufacturing of domestic drugs, and is optimistically expected to increase the value of production by more than 10 billion dollars. This will encourage the domestic pharmaceutical industry to accelerate the innovative research and development of new drugs and enhance the high-end manufacturing capacity, from the perspective of the actual profitability of the industry, it is also expected to increase the output value of the biotechnology pharmaceutical industry, so that from the past, the ratio of the cost of the dream, towards a more profitable ratio of the cost of the benefit and increase the willingness to invest in the capital market.
More Ji-Pu complete professional reviews:Tim Singh steps down, Intel's future direction?
Related news link: News
Nov W2|Big Health News Highlight: "Trump's Rise Again" Pharmaceutical Industry's Impact on Two Key Issues, List of Beneficiaries
With Trump back in the White House, the market has named the revamping of Obama's health care policy as one that will favor drug price adjustments and accelerate the development of the famous pharmaceutical companies. Legal entities and industry players have also assessed that the new administration's focus on manufacturing in the U.S. will be beneficial to pharmaceutical companies that have manufacturing and R&D bases in the U.S.
Ji-Pu Viewpoint:
With the U.S.-China game and geopolitics, the U.S. biotech-pharmaceutical industry and China's CDMOs have been on the path of decoupling, with 2025 expected toTrump's New Deal America First and Made in AmericaOn the premise of protectionism in the U.S., the U.S. domestic production and research and development of pharmaceutical companies, reduce regulatory policy to expand the development of innovative drugs in the U.S., trading mergers and acquisitions or expanding overseas sales and foreign exchange earnings are expected to increase significantly, and in order to effectively control the burden of health care in the U.S., the accelerated expansion of the choice of prescription medicines, increase the health care industry, transparent competition and transparency in the pharmaceutical industry, policies such as the promotion of the market of generic drugs and biosimilar medicines to meet the new opportunities, so the establishment of U.S. factories in Taiwan or the related pharmaceutical supply chain that is beneficial to the U.S. pharmaceutical industry will be expected to meet the new opportunities. As a result, new business opportunities are expected to arise for Taiwan pharmaceutical companies with U.S. plants or related pharmaceutical supply chains that are favorable to the U.S. pharmaceutical industry.
Related news link: News
Oct W4|Big Health News Highlight: Poray spends another $870 million to acquire U.S. brands of rare disease drugs
The Board of Directors of PuraPharm has approved the acquisition of all shares of Pyros, a U.S.-based rare disease drug development company, to acquire Pyros' key branded drugs for rare diseases at a closing price of US$27.25 million (approximately NT$870 million). The branded drug portfolio for rare diseases acquired through the acquisition will not only expand PuraPharm's global sales of medicines in the U.S. market, but will also reduce the original concentration of brand-name medicines and help to transform the branded channel with patent protection advantages. In addition to expanding the product pipeline in the U.S. market, the acquisition will also reduce the concentration on generic drugs and help transform the branded channel with patent protection advantages.
Ji-Pu Viewpoint:
From a channel and product perspective, the acquisition has two major strategic highlights for Porui's operations: 1. Porui's USL (Upsher-Smith) has an advantage in the pediatric neurology channel, and the expansion of its related product portfolio in terms of channel reinforcement will help increase gross margins and revenues, as well as raise the competitive barriers; 2. The nature of the CDMO OEM business relies on the sales capacity of branded channel partners. Under the premise of introducing exclusive patented high margin branded drugs such as 505(b)2 new drug portfolio, it is more capable of sustaining long-term drug market share and stable profitability of drugs. After all, under the price competition pattern of generic drugs, the transformation and integration of the marketing brand and competitive barriers of channels are the only way to secure a firm footing under the competition of pharmaceutical products.
Related news link: News
Porui Acquires U.S. Brands for 870 Million Dollars|MaxOffice|
Oct W2|Big Health News Highlight: Artificial Reproduction Bill will be sent to the Legislative Yuan by the end of the year.
The draft amendment to the Artificial Reproduction Act focuses on opening up the application of single women, same-sex couples, and surrogate mothers. According to the Minister of Health and Welfare, the National Health Administration is expected to send the draft to the Ministry of Health and Welfare by November, promising that the draft can be sent to the Executive Yuan by the end of this year at the earliest.
Ji-Pu Viewpoint:
Elon Musk Says Having Kids Will Save Human Civilization! Current TaiwanArtificial Reproduction Actstipulating that only couples can practise artificial reproduction (i.e. in vitro fertilization).Single Women Cannot Buy or Use Sperm in TaiwanFrom the industry's point of view, the amendment of the Taiwan Artificial Reproduction Act will have the opportunity to promote the industrialization of reproductive medicine services and more internationalization, as well as the upgrade of IVF technology and genetic testing. From an industry perspective, the amendment of Taiwan's artificial reproduction law will have the opportunity to promote the industrialization and internationalization of reproductive medicine services, as well as the upgrading of IVF technology and genetic testing for genetic or reproductive genes. From the perspective of genetic eugenic healthcare, in vitro fertilization (IVF) includes preimplantation embryo testing, prenatal fetal testing, postnatal newborn testing for potential genetic diseases, and genetic testing for all-around care from IVF to newborns, as well as improving service content and extending the service chain to include international cooperation in surrogacy and other business opportunities.
Related news link: News
Sep W4|Big Health News Highlight: Dr. Benson Considers Sale!
World-renowned contact lens maker Bausch + Lomb (B&L) is rumored to be discussing a possible sale to separate itself from its debt-ridden parent company Bausch Health, with private equity as the "most likely" buyer.
Ji-Pu Viewpoint:
Competition in the international contact lens supply chain continues to intensify, with Europe and the U.S. still dominating the top four suppliers. Although Johnson & Johnson, Alcon, CooperVision, and Bausch & Lomb have brand and channel advantages, their global market share is on a downward trend, so how to continue to capture market share is still the core of each company's operation. How to continue to capture market share and increase revenue is still the core of each company's operation. Recently, Dr. Len was forced to consider a sale due to the parent company's debt problems, which has made manufacturers who are already eager to compete in the market eyeing the company. This time, due to anti-trust regulations, the company was not able to snatch the company, but it remains to be seen whether private equity intervention will create a reshuffle in the international contact lens supply chain or introduce an opportunity for the Asian contact lens supply chain to participate in the market.
Related news link: News
Parent company in financial trouble Dr. Rumor is exploring a sale|Freelance Business
Sep W1|Big Health News Highlight: Porui Group's merger and acquisition is a big hit with big names
Taiwan's biotech industry is playing in the International Cup. Under the leadership of Chairman Sheng Po-Hsi, Porui (6472) Group has been expanding its business by continuous mergers and acquisitions, including the four kings of the biotech industry, such as Zhao Yutian, who have been cooperating with him one after another. This time, the alliance with Taifu (6541) through a share swap shows that Sheng's management strength has been praised even by Ruentex Group President Yin Yanliang.
Ji-Pu Viewpoint:
Taiwan's biotechnology and medical industry is still in a small and beautiful (business scale is not large), as well as the international market share and volume of foreign sales (exports do not account for a large proportion of foreign exchange earnings) is not enough of the dilemma, and mergers and acquisitions strategy to play the International Cup seems to be one of the ways to break the game, with the mergers and acquisitions to show the benefits of the fermentation does not rule out the possibility of successively driving other biotechnology and medical companies to follow up the recent whether it is a good Shida, the United States, or Taiwan Kang have said that they are starting to mergers and acquisitions of the intentions of the heart, and long-term view, if we can improve international sales channels and supply chain integration will have the opportunity to enter a larger scale of growth opportunities. In the long run, if we can improve the international sales channel paving and supply chain integration, we will have the opportunity to enter a larger scale of growth opportunities.
Related news link: News
Porui Group Mergers and Acquisitions Big Critics Applauded|Economic Times
Aug W4|Big Health News Highlight: Fuso announces $1 billion investment in two Diamond Biotech subsidiaries to capitalize on biomedical growth opportunity
Fubon Financial Holding (2881-TW) announced on behalf of its subsidiary, Fubon Life, that the Board of Directors has resolved to invest in Diamond One and Diamond High-Tech, two subsidiaries of Diamond Biotech (6901-TW), with the estimated investment amount not exceeding NT$1 billion each and not exceeding the investee's paid-in capital amount of NT$25%, with the investment to be made in a single subscription and in separate issues. Diamond One and Diamond Hi-Tech are venture capital funds with an estimated size of NT$5 billion to NT$8 billion each. Diamond One will focus on investing in the biotech and medical industry during the expansion and growth phase, while Diamond Hi-Tech will focus on investing in mid-to-late stage biotech and medical technology and product companies.
Ji-Pu Viewpoint:
The pool of capital is the bloodline of an enterprise and the backbone of enterprise resource integration. In addition to Diamond Biotech, Billion Biotech (Fubon), China Development Capital, Taisan Biotech Fund, and Anfo Fund in the field of biotech-pharmaceutical investment in the domestic market, Fubon Life has increased its investment in Diamond Biotech and has introduced cross-border funds from overseas, which will lead the integration of technological and industrial resources of various parties, and help accelerate the development of biotech-pharmaceutical products or services by linking up capital. In addition to the integration of technology and industrial resources from various parties, the linked capital will help accelerate the development of biotechnology and pharmaceutical products or services, and further the cooperation in product licensing or listing.
Related news link: News
Aug W2|Big Health News Highlight: AI/ML drug screening company Exscientia merges with Recursion
Exscientia brings its sophisticated chemical design and automated small molecule synthesis technologies to Recursion, contributing to scale-up biological discovery and translational capabilities.The combination of AI/ML screening company Recursion and Exscientia will create a company with $850 million in cash and 10 clinical trials to be conducted over the next 18 months.
Ji-Pu Viewpoint:
Everything is AI/ML, and the industrial demand for AI/ML drug screening comes from the high cost of new drugs on the market, long R&D cycle, huge R&D costs, very complicated and expensive clinical trials, and unpredictable failure rates, which directly increase the cost of drug development. Therefore, many companies continue to invest in AI drug screening, utilizing big data, cloud computing, machine learning and other artificial intelligence technologies to assist in drug discovery, drug development, clinical trial management and other aspects. With the global AI/ML innovators raising more than US$12.5 billion in funding, new AI/ML screening companies such as Xaira Therapeutics (expert-rich, US$1 billion in funding) and Formation Bio (Open AI-backed, US$372 million) have set off a new wave of AI/ML drug screening boom.
Related news link: News
Recursion and Exscientia Merging to create artificial intelligence biotechnology|biospace
July W4|Big Health News Highlight: Sunshine raises stake in Minda Medical
Sunshine continues to increase its shareholding in Minda Medical, acquiring 336,000 shares in the open market, increasing its shareholding from 15.06% to 16.38%. Sunshine is actively expanding its eye health layout. Its medical service company, Chuan Yang Vision, has already cooperated with medical institutions to develop autologous serum ophthalmic solution, which is mainly used to improve dry eyes and restore corneal damage, etc., and will invest in the research and development of other eye health products and eye examination equipment in the future.
Ji-Pu Viewpoint:
In addition to the contact lens business group, Vision Sun has also been actively involved in eye health, such as acquiring shares in Minda Medical, developing autologous serum eye drops to improve dry eye, corneal damage recovery, other eye health products, eye examination equipment, etc. With the star endorsement of the contact lenses and the increase in brand awareness, Vision Sun has also begun to lay out the peripheral ophthalmology eco-circle product line, and will not rule out the possibility of integrating the Group's resources to build brand channel competitiveness in the future. The future integration of group resources to build brand channel competitiveness. Taiwan's contact lens group is expected to continue to increase the international market share of Taiwan's contact lenses due to the rising demand for silicone rubber in Asia, the rise of presbyopia and anti-blue light functional lenses, and the cost-effectiveness of AI automated manufacturing and standardized mass production.
Related news link: News
Sunshine to increase stake in Minda Medical|Business Times|Sunshine
Jul W2|Big Health News Highlight:5 M&A, 4 Pharma Licenses, Biotechs Going International
In the first half of this year, the biotech industry witnessed five mergers and acquisitions (M&A), including the US$210 million (NT$6.6 billion) acquisition of Upsher-Smith (USL), a century-old U.S. pharmaceutical company, and the US$30 million acquisition of Emergent BioSolutions' aseptic formulation plant. NanoMed acquired Millennium, a U.S.-based company, for US$25.65 million (NT$800 million). TAYO acquired Synchem, a North American CDMO company, to accelerate its expansion in the U.S. market in conjunction with the five-fold expansion of ADC (Antibody Drug Complex) production capacity in the third quarter. MTS acquired the Thai subsidiary of Teva Pharmaceuticals, the world's largest scientific pharmaceutical company, and its business.
Ji-Pu Viewpoint:
Taiwan's biopharmaceutical industry wind vane: M&A integration benefits + drug licenses with sales indicators, is the focus of the investment market. With Taiwan's CDMO high-end manufacturing successively inserted into the U.S., can be seen that the U.S. Biosafety Act does bring the U.S. market opportunities for reordering, as well as the U.S. domestic inflation under the shortage of drugs business opportunities, and expand the license market is expected to be a breakthrough for the sales of drugs, Taiwan's biotechnology and pharmaceutical industry is in need of the wind to the sales results to confirm the approval of the drug in the medical needs of the market to validate the business opportunities, the 7/24 Bio Asia Conference (Bio Asia) debut, CDMO and strategic sales indicators are the focus of the investment market. Bio Asia will be held on July 24th, and the CDMO and strategic cooperation layout will be one of the highlights.
Related news link: News
Jun W4|Big Health News Highlight:Porui Expands U.S. Footprint with Another M&A, Buys Sterile Preparation Plant for $960 Million
Porui Pharma announced today that it will acquire Emergent BioSolutions' aseptic manufacturing facility in Maryland for US$30 million (approximately NT$969.3 million). The acquisition will be Porui's third manufacturing facility in the U.S., and will significantly strengthen the process technology and development capabilities of its global contract manufacturing operations (CDMOs).
Ji-Pu Viewpoint:
The new version of the U.S. BIOSECURE Act (BIOSECURE Act) draft, is gradually building biotechnology pharmaceutical defense, Chinese CDMO enterprises to receive orders under the headwinds, Taiwan CMDO is also expected to meet a share of the opportunity to get a piece of the pie. Currently, Porui and Taiyao have set their flag in the U.S. through mergers and acquisitions, while Yungshing, North Star Pharmaceutical-KY, Taifu, Handa and EWI have already set up their supply chains in the U.S. Among them, Porui has the largest business volume in CDMO, which covers the fullest range of pharmaceutical technologies, from small molecule medicines to protein medicines, and the current acquisition of a sterile preparations plant will expand four sterile injection production lines, as well as freeze-drying, glass vial filling, and pre-packed syringe filling, which will provide a comprehensive range of manufacturing technologies. The acquisition of the sterile preparation plant will help strengthen the CDMO business expansion of small molecule drugs, peptide drugs and protein drugs, and the benefits of CDMO order intake are expected in the future.
Related news link: News
Jun W2|Big Health News Highlight: Regenerative medicine law read for the third time...writing a new milestone...
After ten years of hard work, the regenerative medicine double law (6/4) was finally passed in the Legislative Yuan for the third time, in the future, non-medical organizations are not allowed to perform regenerative medicine, violators will be fined up to NT$20 million, and regenerative technology should be carried out before the implementation of the completion of the human body test, but EnCi treatment is exempted from the completion of the human body test. The dual laws on regenerative medicine are in place at once, making Taiwan the third Asian country, after Japan and Korea, to enact a special law on regenerative medicine, which is a milestone in Taiwan's medical progress.
Ji-Pu Viewpoint:
Taiwan has benefited from the enactment and liberalization of domestic laws and regulations, which not only safeguard the rights of patients to receive advanced treatments and ensure the safety and quality of regenerative medicine performed by qualified medical institutions, but also provide Taiwan with a legal basis for regenerative medicine research and development, cellular agent technology management, and cellular source management to ensure that the competent authorities have a basis for supervision and control, which will be conducive to the industry's experience in the validation of cellular agent technology and clinical efficacy data, and will also enhance agent technology innovation and the quality of cellular agents to enhance international competitiveness. The regenerative medicine for cancer is no longer limited to patients whose "standard treatments are ineffective" and "end-stage solid cancer", but all cancer patients can use the regenerative medicine for cancer approved by the Ministry of Health and Welfare, and how to strike a balance between safety, therapeutic efficacy, treatment cost, and industrial development is a key issue. How to strike a balance between safety, efficacy, treatment cost, and industry development, and how to positively develop Taiwan's regenerative medicine industry so that innovative technologies can be applied on the ground and seize the first opportunity to gain a foothold in the international arena, or to spend healthcare resources and return home without major breakthroughs in healthcare, will still be a test of time for the wisdom of all parties involved.
Related news link: News
Jun W1|Big Health News Highlight: Invisalign Rookie Wins Manufacturing Processes
Preliminary statistics, in 2024, the total monthly production capacity of contact lenses in Taiwan will reach 288 million lenses, and 32 million lenses in China, which is only 11% of the total capacity in Taiwan. Because contact lenses do not rely on equipment can be mass production, the need for a long time to adjust the production and parameters, the experience of Taiwan, we must mass production for more than 5 years to achieve the level of wearing comfort, reduce costs is one of the keys to victory, the new generation of the rise of the crystal, looking at the Falcon, are the background of the electronics industry, through the high level of automation and cost reductions, led to the sharp growth in recent years, profitability.
Ji-Pu Viewpoint:
Contact lenses have become a necessity in daily life, and the trend of wearing contact lenses is emerging! Contact lenses is a consumer terminal marketing and brand-oriented market, the new listing aspirations and themes, with sales activities and consumer word-of-mouth is the key to boost sales, Taiwan as Japan and China's key brands supply chain, with AOI automatic optical inspection and automation degree of performance, production efficiency, yield and cost advantages, and win over Korea and China, the initial observation of contact lens foundry potential order volume increase, but will continue to pay attention to the factory expansion. Initial observation of contact lens foundry potential order volume increase, have increased the expansion of factories, but will continue to pay attention to the mainland and Japan's actual growth of the changes and force.
Related news link: News
Invisalign Rookie Process Winner|Business Times|The Invisalign Rookie
MayW3|Big Health News Highlight: U.S. House of Representatives passes biosafety bill that would restrict WuXi PharmaTech and other Chinese companies if it goes into effect
The new U.S. Biosafety Act was passed by a margin of 40-1, and has yet to be voted on by the full U.S. House of Representatives and the full U.S. Senate, and then signed into law by U.S. President Joe Biden. Under the Act, U.S. pharmaceutical and healthcare companies will be restricted from doing business with Chinese biotechnology companies including WuXi AppTec, Huada Genetics, Huada Smart, and WuXi Biologics.
Ji-Pu Viewpoint:
The new version of the Biosecure Act of the U.S. Congress is still in the process of reviewing and legislating, if it is hastily decoupled from China's production supply chain, it may affect the supply of drugs to millions of patients in the U.S., and the replacement of the production partner needs to go through the process of test runs and production validation during the transfer of the plant as well as the re-passing of the U.S. FDA's regulatory approvals and the increase in costs, etc., which is expected to take 2~8 years. It is expected to take 2~8 years. With the continuation of the trade war between the U.S. and China and the U.S. presidential election, it seems that the bill is heading towards its formal entry into force, and it is currently observed that a number of international CDMO manufacturers such as Lonza, Patheon (Thermo Fisher Scientific), and Samsung Biologics have already begun to lay their groundwork, and it is expected that they will see tremendous growth opportunities.
Related news link: News
AprW4|Big Health News Highlight: TaiGen*-KY's new influenza drug succeeds in Phase III blinding, effective in treating influenza
TaiGen*-KY (4157) has completed its Phase III clinical trial of influenza antiviral drug TG-1000 with its partner in Mainland China, Healthwon Pharmaceutical Group. Healthwon expects to submit an application for registration of TG-1000 in Mainland China in the second half of 2024, with the goal of launching the drug in Mainland China one year later, and TaiGen will also conduct overseas licensing in Europe, the United States, and other Asian countries. TaiGen will also conduct overseas license negotiations in Europe, the United States and other Asian countries.
Ji-Pu Viewpoint:
Anti-influenza virus is the highlight of the public health system every fall and winter, anti-influenza drug resistance or viral mutation is the main cause of drug failure, the research and development of new anti-influenza drugs is still the focus of the market, which selects different mechanisms of action of the drug combination is one of the strategies for the future of the anti-influenza virus drugs, TaiGen's past antibiotic Nemonoxacin (Nemonoxacin) has been authorized in 35 countries, with the experience of new drug authorization and cross-border partner channels. TaiGen's past antibiotic Nemonoxacin has been licensed in 35 countries, and with its experience in new drug licensing and multinational partner channels, the future business opportunities of TG-1000 will be able to observe the payment of milestones, the sales profit from the launching of the drug (estimated to be up to 11%), and overseas licensing fees in Europe and the United States.
Related news link: News
AprW3|Big Health News Highlight: Sinochem to go off the market, restructuring holding to be listed again
Another company with stock number "01" disappears! The pharmaceutical industry has entered into a generational change. In order to enhance the overall business strategy integration, Sinochem Pharmaceutical intends to establish Sinochem Investment Holdings by a 1 for 0.5 share swap. Upon completion of the share swap, Sinochem Pharmaceutical will terminate its listing and be listed by Sinochem Holdings.
Ji-Pu Viewpoint:
The value of Porui's M&A strategy has been favored by the market. In addition to the technology industry's shareholding or M&A in the biotechnology industry, the generational change of pharmaceutical companies is also gradually forming another wave of the new trend of strategic transformation, and recently, many index companies, such as YSP, Sinochem, and Kencho Shinyuan, are carrying out generational change or initiating the cultivation of a new generation of successors or TAYA or Taikang have announced that they will not rule out overseas mergers and acquisitions, etc. As the competition for the industry intensifies at both the international and inter-regional levels, it is a challenge to determine how decision-makers can upgrade and transform the industry and enhance the comprehensive efficiency of the industrial chain. As international and regional competition in the industry intensifies, it is a test for decision makers on how to upgrade and transform the industry and enhance the comprehensive efficiency of the industrial chain, and also an opportunity for Taiwan's pharmaceutical industry to seize a place in the international or regional arena.
Related news link: News
AprW2|Big Health News Highlight: M&A Fever: Biotech Equity Moves the Storm
According to statistics, at least ten companies have been merged, acquired shares or introduced strategic investors in recent years. The hottest drama this year is the proposed acquisition of 25% to 35% of Tianliang's shares by Tingyao Health and Taipei Zuoyu at NT$16.8 per share, while Wuding has been acquired by Kecheng and Sanyang, and microbiology testing-based Qixing Technology has introduced strategic investors such as Bao-rui, Medical Young and Lescience.
Ji-Pu Viewpoint:
With the recent high stock market and the influx of capital in the market, the technology industry's shareholding in or merger and acquisition of the biotechnology industry has gradually formed another wave of new trends in the development of biotechnology and healthcare. In addition to indicating that the technology industry recognizes the future prospects of the development of the biotechnology and healthcare industry, some value-based biotechnology and healthcare companies are gradually surfacing in the market. Continuous blood glucose monitoring equipment is a high-tech, high-margin product, which is expected to enhance the profitability and gross profit of Taiwan-based companies and drive a new wave of operational energy.
Related news link: News
Merger and Acquisition Fever: Biotech Equity Move|Institute of Business Times
MarW3|Big Health News Highlight: Liver Disease Prevalence: Fatty Hepatitis Drug Market to Exceed $48 Billion by 2035
Madrigal Pharmaceuticals has received FDA approval for the world's first NASH treatment. The oral thyroid hormone receptor (THR) agonist, called Rezdiffra, helps maintain liver stability and has been shown to improve lipid metabolism. It has been shown to improve lipid metabolism and will be available in the U.S. at a list price of $47,000 per year of therapy.
Ji-Pu Viewpoint:
Madrigal Pharmaceuticals' new steatohepatitis drug, resmetirom (trade name: Rezdiffra), was approved by the U.S. FDA on March 15, 2024 as the world's first steatohepatitis drug. It is indicated for the treatment of patients with metabolic dysfunction-associated steatohepatitis (MASH) with moderate to advanced hepatic fibrosis at a cost of $47,000 per year of therapy. Competitive steatohepatitis drugs, there are also clinical studies of GLP-1 drug (the U.S. FDA has not yet approved), clinical trials indicate that GLP-1 drug can treat the early stages of steatohepatitis, for GLP-1 drug price ($15,000 per year of treatment) for the Rezdiffra 1/3, with more and more clear metabolic dysfunction pharmacology, in addition to solving the human liver in the accumulation of fatty tissue caused by the disease. With increasing clarity on the pharmacological mechanisms of metabolic dysfunction, the steatohepatitis drug therapy market is expected to see high growth gains, in addition to addressing the disease caused by the accumulation of fatty tissue in the human liver.
Related news link: News
MarW2|Big Health News Highlight: National Security Concerns Sweep Into China's Pharmaceutical Industry
National security concerns in the U.S. are affecting popular weight-loss drugs and diabetes treatments. The Homeland Security Committee of the U.S. Senate voted to approve the Biosecure Act on June 6, 2012, which could restrict U.S. dealings with biotech companies from mainland China such as U.S. Genetics and WuXi WuXi AppTec in order to reduce reliance on mainland China, which produces most of the active ingredients in the base of some weight-loss drugs.
Ji-Pu Viewpoint:
The U.S. biosafety bill accelerates the de-Chinaization, the three keys to CDMO development are advanced technology capability, scale and trade relations in the industry chain. With the continuation of the U.S.-China trade war, the relevant U.S. laws or drafts have hindered the cooperation between China and the U.S. in the field of biotechnology and pharmaceuticals, and the impact of the level of the expansion step by step, the global biotechnology and pharmaceuticals CDMO OEM business is mainly concentrated in the U.S., Europe, as well as China and South Korea. Previously, Novo Holdings acquired Catalent, a U.S. pharmaceutical contract manufacturer, for US$11.5 billion, and Samsung Biologics of Korea deepened its cooperation with global international pharmaceutical companies. As the risk of Chinese CDMO enterprises receiving orders gradually rises, CMDO, a Taiwan-based company, will also welcome the opportunity to get a share of the pie.
Related news link: News
U.S. Anxiety Sweeps Into China's Pharmaceutical Industry|Institute for Business Times
MarW1|Big Health News Highlight: Taiwan's pharmacy chain is the king of stores! Youquan to be listed on the Taiwan Stock Exchange in March to explore the blue ocean of business opportunities for aging and silver hair.
In addition to providing health insurance prescriptions, health care nutritional foods, women's and infants' products, and health care products to meet the one-stop shopping needs of customers of all ages, Youquan Pharmaceuticals is currently the largest pharmaceutical chain in Taiwan, and it will also actively lay out its market for pets and recreational ageing. According to Taishin Investment Consulting, the trend of chain drugstores in Taiwan is very strong, and there is still a lot of room for growth in the Taiwanese chain drugstore market. It is estimated that the number of chain drugstores in Taiwan in 2050 will grow by more than 6,000, and the proportion of chained drugstores will be more than 50% compared to the 2022 growth, with a potential business opportunity of more than $205 billion, and all major chain brands will be benefiting from the growth.
Ji-Pu Viewpoint:
Chain drugstores on the counter wave appeared (Youquan and Noble), the exhibition of stores to show the best tricks, from the tide of consolidation to the tide of capital to move, in the chain drugstore chain capital, is expected to enter the warring states era of the drugstore channel, competition will be more heated, from the past, mainly focusing on women's and children's health products, medical materials, OTC health care prescriptions and cosmetic channel characteristics of the type, and gradually across the one-stop shopping for health care-oriented drugstores. In the post-capitalization era, how to earn management money to build profitable stores is particularly important, and it is expected that the Taiwan pharmacy channel market will move towards 50% chain, and from the past long-term development trend of the retail industry, the dividends of the ultimate chain pharmacy channel market will gradually be formed by the 2 strong division of the situation.
Related news link: News
Feb W4|Big Health News Highlight: Taiwan's Biotech M&A Fundraising Opportunity: J.P. Morgan Chase Explores the Path to Taiwan
Taiwan's biotechnology mergers and acquisitions to raise business opportunities, so that large investment banks to step up the pace of layout, the investment bank JP Morgan Chase took the lead in Taiwan to explore the road, in the aftermath of the epidemic in the new crown, has seen at least 60% to 70% in the past in the electronic technology industry center of gravity to the biotechnology and health care industry to move, especially the combination of AI health care will also be the mainstream trend.
Ji-Pu Viewpoint:
Taiwan's biopharmaceutical or medical equipment development to date, the industry scale driven by revenue expansion has been a soft spot in the company's growth and development of mergers and acquisitions is one of the important strategic tools in the market scale of the business project selection and linkage of the capital market, how to create a comprehensive effect of operational integration has always been a deep thought of the entrepreneurs in the health care year after year, the decline in gross margins and the price of the products to compete in addition to the hope of innovative products to go public to extend the growth momentum in the upstream and downstream industry chain integration or the introduction of strategic investors to create opportunities for upgrading and transformation is still looking at the insights behind the business opportunities. In addition to hoping for innovative products and continued growth momentum, the integration of upstream and downstream industry chains or the introduction of strategic investors to create opportunities for mergers and acquisitions to upgrade and transform is still the focus of insight into the business opportunities behind.
Related news link: News
Feb W1|Big Health News Highlight: TBMC, Firmware USA form strategic alliance to build new CDMO model for biotech industry
Taiwan Biomedical Manufacturing Corporation (TBMC) and National Resilience signed a strategic alliance agreement for technology transfer and investment. TBMC will acquire licenses for five advanced manufacturing processes from National Resilience in biologics, vaccines, mRNA nucleic acid drugs, cellular therapies, and gene therapies, which will accelerate the connection of the domestic nucleic acid drug industry with the global market and facilitate in-depth integration with National Resilience's global manufacturing bases in the future. TBMC will accelerate the connection between the domestic nucleic acid drug industry and the global market, and will facilitate in-depth integration with National Resilience's global manufacturing bases in the future, thereby enhancing its global strategic position.
Ji-Pu Viewpoint:
Advanced biotechnology processes for mass production of biologically active molecules such as therapeutic antibodies, proteins, mRNA nucleic acids, or cellular drugs are considered to be the key manufacturing technologies for mass production of biopharmaceuticals. The establishment of gene banks and cell factories through high-throughput gene synthesis, gene transfer, and gene editing technologies, or the creation of cell synthesis factories by genetically modifying cells to produce high-quality biomolecule raw materials (pharmaceuticals) with commercial value on an economic scale and at low cost is an important trend in the biotechnology manufacturing revolution. Inheriting the advantages of U.S. innovative IP and Taiwan's manufacturing capabilities, we are in the process of developing a new drug development strategy that incorporates production cost control.
Related news link: News
Jan W5|Big Health News Highlight: New Investment Appearance: New Drugs and Medical Materials Most Favorable
Layout of technology groups in the biotechnology industry has become a trend, the single gang technology industry also set off a trend, which is the most favored new drugs, medical materials field, Charming, ZhenYao, TFN, ChangHua, respectively, in the How to show, Taiwan biomaterials, Dharma, look at the Falcon, and the light cover, the main body, ShuoWo aimed at Renegade thick, Polaris, Yaguo biomedicine.
Ji-Pu Viewpoint:
The technology industry has continued to invest in the biotechnology and medical industry, with investment covering a wide range of areas such as operating room equipment, medical consumables and dressings, contact lenses, blood glucose machines, ophthalmic instruments, handheld ultrasound equipment, pharmacy chains, genetic testing, medical management consultants, medical monitors, minimally invasive laparoscopic surgical materials, hospitals, exoskeletal robots, medical material foundries, healthcare or cosmetic and skin-care products, AI-assisted diagnosis software, cell derivatives, foundries or new drug development, with the supply chain integration of medical and electrical equipment and devices as the most favored products from the investment perspective. software, cell derivatives, foundry or new drug development, with supply chain integration of medical electronics and instrumentation medical devices being the most favored. From an investment perspective, after years of incubation, many small golden chickens with good profitability and high gross margins have been hatched, except that the growth of individual revenue scales in comparison to technology companies is still insufficient, and future observations will focus on the benefits of grouping and the response of the capital market.
Related news link: News
Jan W4|Big Health News Highlight: HHS announces the launch of NGS in May this year at the earliest.
The Health Insurance Administration plans to pay for 19 types of cancer tests, which will be launched in May this year at the earliest, and each cancer patient and each type of cancer can use the health insurance to pay for one time in their lifetime, and it is expected that 6~1 billion yuan will be budgeted annually. 2022 health insurance data analysis shows that 830,000 patients will be diagnosed with cancer in that year, and 39.2 billion yuan will be spent on medication, and the percentage of targeted medication will be the highest, which accounts for 61.7% of the total. Currently, health insurance covers 26 types of targeted drugs, corresponding to 11 gene loci, for the treatment of 10 types of cancer, but the coverage does not include NGS, only single locus testing, which is about $200 million a year, or PCR testing, which is about $400 million a year.
Ji-Pu Viewpoint:
Cancer genetic testing is an important tool toward accurate drug use and companion diagnosis. With the need for treatment, more and more cancer drugs require multilocus NGS, and early cancer screening with simultaneous detection of multiple cancers is also an important development trend. Health insurance coverage of NGS will contribute to the development of disease, gene and clinical medical database applications, and drive business opportunities in cancer gene testing for Taiwan-based companies such as InFocus Digital Genetics, Goldman Sachs, and Wise.
Related news link: News
Jan W3|Big Health News Highlight: Weight Loss Drug Craze Continues
Two major international pharmaceutical companies, Novo Nordisk and Eli Lilly, set off a global sales boom of weight-loss drugs last year, and the domestic biomedical community has also smelled the business opportunities, including the United States, Polaris Pharmaceuticals, Tai Zong, You Hua, Immunity Kung Fong, Kang Kang, and other companies have gradually entered the development and marketing of related drugs. Yulong is also the sole supplier of Novo Nordisk's weight loss drug injection parts in Taiwan.
Ji-Pu Viewpoint:
It is optimistic that the global scale of GLP-1 weight-loss drugs in 2030 will reach US$100 billion. Taiwan's Polaris, Immunogonfo, and Yulong have already seized the business opportunity of GLP-1 weight-loss drugs. Currently, the original pharmaceutical company, Novo Nordisk, is actively developing GLP-1 weight-loss drugs for other extended indications, including heart disease, steatohepatitis, chronic kidney disease, and even lowering the risk of cancer, and with the release of the subsequent clinical trial data, it will surely set off another wave of GLP-1 weight-loss drugs around the globe.
Related news link: News
Jan W2|Big Health News Highlight: New Business Opportunity for Medical Beauty...Laxation of Exosome Controls
The Ministry of Health and Welfare (MOHFW) has previewed the draft amendment, which is expected to open up the possibility of obtaining raw materials from the human body for cosmetic products in July. The Food and Drug Administration (FDA) of the Ministry of Health and Welfare (MOHFW) has recently announced that it will amend the draft of the "List of Prohibited Ingredients for Cosmetics", and after collecting opinions for 60 days, it is expected to open up exosomes of human origin to be used as raw materials for cosmetics on July 1st. The Ministry of Health and Welfare (MOHFW) announced on December 13 last year that it would amend the draft of the "Cosmetic Prohibited Ingredients List" by adding a new note to the list of cells, tissues, or products of human origin that are prohibited from being used: "Those that have been examined and approved by the central competent authority in respect of individual exosomes of individual traders are exempted from the restriction". The preview of this executive order has aroused the concern of the biotech industry.
Ji-Pu Viewpoint:
With the rapid development of global Exosome research and industry, Exosome in addition to new drug therapy research by the international attention, cut into the international cosmetic ingredients raw materials to obtain INCI new raw material certification is also one of the options to open up business opportunities, at present the international Europe, the United States and Japan have approved the human source of mesenchymal stem cells Exosome cosmetic ingredients, with the gradual relaxation of the regulations in Taiwan, will also be conducive to With the gradual relaxation of Taiwan's laws and regulations, it will also be beneficial for Taiwan manufacturers to open up new horizons in the international cosmetic and nutraceutical ingredients.
Related news link: News
Jan W1|Big Health News Highlights: CHPTA Established to Accelerate Health Insurance Coverage for New Drugs
In the past, when new drugs were covered by health insurance, the new drug had to obtain a food and drug license before applying for health insurance coverage from the Department of Health Insurance (DHI). Under the parallel submission mechanism, new drugs can apply for a drug license and health insurance coverage at the same time, and it is estimated that the drug can be covered six months after obtaining a drug license; as for new drugs that do not undergo the parallel submission mechanism, they can be covered in about 12.5 months after the original acceptance of the health insurance coverage, which has been shortened to 10 months. Next year, a total of five major new drug categories will be included in the parallel review mechanism, including those that have been recognized by the FDA as requiring priority review and accelerated approval, those for pediatrics or a small number of serious diseases, or those that have not yet been marketed internationally at the time of the domestic application for review and registration.
Ji-Pu Viewpoint:
The Department of Health Insurance (DHI) of the Ministry of Health and Welfare (MOHW) announced that the Center for Health Policy and Medical Technology Evaluation (CHPME) has officially started to operate the parallel submission mechanism for new drugs and the temporary payment mode of health insurance, which will significantly shorten the time for new drugs to be included in the health insurance from an average of 13 months to 6 months, and will also benefit patients with urgent medical needs for the 2 years of temporary payment of health insurance for specific new drugs, such as lung cancer, cholangiocarcinoma, leukemia, and the newest cellular therapeutic product, CAR-T. This will accelerate the pace of new drug launches and new drug sales in Taiwan and expand the accelerated development of the biotechnology and pharmaceutical industries.
Related news link: News
Dec W4|Big Health News Highlights: FDA approves two gene therapies for sickle cell disease
The U.S. FDA this month approved two gene therapy treatments for sickle cell disease, Vertex and CRISPR Therapeutics' Casgevy.TM The company's products are available in a wide range of colors, including: black, red, yellow, and bluebird bio's Lyfgenia. TMWill gene therapy have long-term side effects? Only time will tell. Both companies plan to conduct 15-year follow-up studies to ensure the long-term safety of the treatments, and the FDA has said that patients using the treatments will be screened for susceptibility to cancer for the rest of their lives. If gene therapy is found to be safe after a few decades, and the costs come down, then perhaps the time will come when humanity will be free of genetic diseases.
Ji-Pu Viewpoint:
CasgevyTMis the first approved therapy to utilize a novel gene editing technology (CRISPR/Cas9); and Lyfgenia TMIn addition, lentiviral carriers (gene delivery carriers) are used to carry out genetic modification. Gene editing technology new drugs (CRISPR-based medicine) approved and introduced, will undoubtedly set off gene editing technology as a cellular gene therapy for new drug development gold rush, is expected in the future there will be more gene editing technology development of genetic diseases gene therapy may be approved, the U.S. FDA is also reviewing Vertex another transfusion-dependent β-thalassemia CRISPR therapy, the decision is expected to be made on March 30, 2024, the FDA is expected to make a decision on March 30, 2024. The U.S. FDA is also reviewing Vertex's CRISPR therapy for another blood transfusion-dependent beta thalassemia treatment, and is expected to make a decision by March 30, 2024.
Related news link: News
Nov W3|Big Health News Highlight: Lilly invests $2.5 billion in German plant as demand for weight-loss drugs explodes
Lilly announced on 11/17 that it will invest $2.5 billion in a manufacturing facility in Germany to support the growing demand for its diabetes and obesity medicines. The company's first major manufacturing facility in Germany, the new plant will begin operations in 2027 and will be critical in supporting Lilly's supply of incretin. Lilly will also invest $100 million in the German Early Life Sciences Ecosystem, as the biopharmaceutical industry struggles to meet the large and growing demand for obesity treatments.
Ji-Pu Viewpoint:
GLP-1 weight loss drug is an important trend in the development of international new drugs this year, 11/8 U.S. FDA formally approved Lilly GLP-1 weight loss drug (tirzepatide), the trade name Zepbound for long-term weight management, the past to the trade name Mounjaro (formerly approved for the treatment of type 2 diabetes) for the treatment of obesity outside the label, the two strong Novo Nordisk and Lilly competition formally formed, with the two strong manufacturing investment accelerated, it is not difficult to see the explosive growth in demand for GLP-1 weight loss drug, it is expected that in the near future, the demand for GLP-1 weight loss drug explosion. Novo Nordisk (Nordisk) and Lilly (Lilly) competition formally formed, with the accelerated investment in manufacturing of the two strong, it is not difficult to see the explosive growth in demand for GLP-1 weight-loss drugs, it is expected that in the near future there is no other pharmaceutical companies to compete with the state of the weight-loss drug 80% market share will be occupied by the two strong.
Related news link: News
Nov W2|Big Health News Highlight: Is the rise of the weight-loss drug GLP-1 impacting the medical device technology industry?
The intense patient interest in GLP-1 drugs for treating obesity has prompted medical technology companies to take a hard look at their potential impact on demand for products such as weight-loss surgeries, blood glucose monitors and sleep apnea equipment. Mizuho research found that GLP-1 drugs have reduced the value of medical device technology stocks by about $370 billion.
Ji-Pu Viewpoint:
In the thin pen big sales and a number of new clinical data to confirm the efficacy of the promotion of GLP-1 drugs are expected to become a change in the treatment of obesity new options, with a number of international financial analysis of GLP-1 drugs will have a significant impact on the anti-obesity treatment market, from the nature of the overweight is the root cause of many diseases, ranging from heart disease to sleep apnea, if the drug appropriate premise of prevention, to reduce the threat of obesity to human health, the premise of reducing the effectiveness of the patient population base also seems to reflect this subtle change in supply and demand. This subtle shift in supply and demand also seems to be reflected in the effectiveness of reducing the patient population base under the premise of appropriate drug precautions to prevent obesity as a threat to human health.
Delving into Obesity Drugs: What Healthcare Executives Are Saying|medtechdive |
Oct W4|Big Health News Highlights: Garmin Health EcoSystem marks "heart" milestone with flagship ECG model
Garmin Health Ecosystem has reached a new milestone by announcing that the Garmin ECG App has been approved by the Food and Drug Administration of the Ministry of Health and Welfare in Taiwan, and that the five flagship models: Venu 3 Series, Venu 2 Plus, fēnix 7 Pro Series, epix Pro Series, and tactix 7 AMOLED have been opened to the use of electrocardiograms in Taiwan.
Ji-Pu Viewpoint:
With the market share of health smart wearable devices increasing year by year, and the deepening of public concern for heart health, as well as the actual rescue of asymptomatic atrial fibrillation cases, single-lead wrist-worn wearable devices such as Fitbit, Apple Watch, Garmin, and Galaxy Watch have been regarded as a convenient tool for assisting in the diagnosis of asymptomatic or symptomatic atrial fibrillation, and with the support of AI electrocardiogram analysis, the battle for capturing the consumer heart health care market seems to be gradually unfolding. With the support of AI electrocardiogram analysis, it seems that the battle to capture consumers' heart health care market is gradually beginning.
Related news link: News
Garmin's Health Eco-System marks "heart" milestone with flagship ECG model|Taiwan English News
OCT W3|Big Health News Highlight: New Cancer Drugs Fund: $6 Billion in Funding Next Year
The Ministry of Health and Welfare (MOHW) has indicated that the Cancer New Drug Fund (CNDF), which is to be established in 2024 with a scale of 10 billion dollars, will be budgeted from the total amount of health insurance, and will be launched next year as scheduled. The new cancer drug fund's financial resources planning, next year, will first be released to the total amount of health insurance spending, from the total amount of temporary payments budgeted more than 2 billion yuan, new drugs and new technologies budgeted more than 3 billion yuan, adding up to a total of more than 6 billion yuan.
Ji-Pu Viewpoint:
The New Cancer Drug Fund prioritizes new cancer drugs. The inclusion of new cancer drugs in the Medicare benefit review criteria and meeting the eligibility criteria for new cancer drug benefits under Medicare will be the key to the future use of new cancer drugs in the temporary payment or Medicare benefit. The establishment of the New Cancer Drug Fund will also help Taiwan's new cancer drug companies to develop and accelerate the debut of new international cancer drugs and benefit more cancer patients in their treatment choices.
Related news link: News
New Cancer Drug Fund: $6 billion next year|Business Times
OCT W1|Big Health News Highlight: A new wave of listing is brewing in the biotech sector
The new quarterly listing train of the biotech group has started. Starting from the 4th quarter of 2023, a number of domestic biotech group enterprises will gradually apply for listing on the stock exchange, and the industry expects that eight to ten of them are expected to enter the capital market in the coming year, which is brewing a new wave of biomedical listings. The market expects that in the coming year, biotech companies will raise at least tens of billions of dollars to enter the capital market, of which anti-cancer and cell therapy-related new drug research and development companies are the two major highlights.
Ji-Pu Viewpoint:
In order to enhance the competitiveness of the capital market, the Taiwan Stock Exchange (TSE) has successively introduced measures such as new industry classifications, relaxation of the six requirements of the Growth Enterprise Market (GEM), and the expected liberalization of credit trading, etc. In the biotechnology industry, the TSE has set the strategic goal of strengthening the development of the biotechnology and medical industry to reach a market capitalization of NT$500 billion, and doubling its market capitalization to 2% in three years, which will benefit from the issues of licensing, expansion of sales market, and the relaxation of regulations, and will be a key target in the research and development of new cancer drugs, emerging cell therapy and AI high-level medical materials. Benefiting from issues such as licensing, sales market expansion and regulatory loosening, the company will attract attention in the development of new cancer drugs, new cell therapy and AI advanced medical materials.
Related news link: News
Sep W4|Big Health News Highlight: Western Venture Capital Rapidly Withdrawing from China's Biotech-Pharma Industry
Venture capital from the U.S. and other countries is cutting back on investments in Chinese pharmaceutical companies, undermining the momentum of the country's biotech industry, analysts say. Analysts say the retreat reflects China's economic downturn and heightened geopolitical tensions.Venture capital investment in Chinese biotech firms will rise to $19.3 billion in 2021, up from $1.2 billion in 2015, and then fall back to $8.9 billion in 2022, with the entry and exit of the U.S. and other foreign investors playing a major role.
Ji-Pu Viewpoint:
In the past, China's biotech industry has relied on government investment, policy support, and a large healthcare market, and cross-border investment within the U.S. has also been an important source of funding for China's biotech industry. While China's biotech venture capital is rapidly maturing, the financing pipeline is not as large or as mature as that of the U.S., and the current biotech market in China is characterized by the presence of a large number of companies that are all engaged in the same business, as well as the Chinese healthcare anti-corruption overhaul. The action could bring additional pressure and discourage investment, with China's CSI Healthcare Services Index having fallen nearly 20% so far this year by the end of August, according to a Reuters report.
Related news link: News
Sep W3|Big Health News Highlights: New Drug Sales...SmartKing Pancreatic Cancer Drug Tops Last Year's Global Sales Over $10 Billion
The Health Policy Council announced the global sales performance of the top ten new drugs in China. The first place is ONIVYDE, a new drug for pancreatic cancer developed by SmartKing Biotech, which has contributed NT$10.11 billion to the global market last year. In second place was Nephoxil, a new drug for kidney disease developed by Bowen & Fuchsin, with global sales of $7.02 billion last year; and in third place was Besremi, a new drug for rare diseases developed by PharmaEssentia, with global sales of $2.27 billion last year. In addition to announcing the global sales performance of the top ten new drugs, the cumulative sales royalties received by some of the new drug companies in the past three years are also listed. The first place is Zhi Keng with NT$1.64 billion, followed by TaiGen Biotech with NT$1.275 billion, and BaoLin Fortune with NT$1.04 billion in the third place.
Ji-Pu Viewpoint:
Biotech new drug stocks have gradually transitioned from the past Benmonbi to the stage of landing results, from the perspective of sales performance, in addition to the delay in the approval of the drug license and regional distribution cooperation authorization variables, reflecting the approval of the drug license, to the actual price of the drug listed, as well as hospitals to promote the procurement of four to six years before a certain level of sales volume may be achieved, with the global market coverage and medical channel penetration rate, will be demonstrated. With the global market coverage and penetration rate of pharmaceutical channels, the real value of the competition in therapeutic circuit selection and drug efficacy will be realized, and the trend of the strongest becoming stronger is bound to be manifested.
Related news link: News
New drug sales of SmartKing's pancreatic cancer drug takes top spot |Economic Daily News
Sep W2|Big Health News Highlight: Novartis confirms Sandoz spin-off plan, sets October 4 completion date
Novartis confirmed that it is moving forward with the spin-off of its generic and biosimilar division, Sandoz, pending final shareholder approval. If approved, Novartis expects to complete the Sandoz spin-off around October 4th. The spin-off will allow both companies to focus on maximizing value for shareholders, as the past spin-off of its ophthalmology business, Alcon, helped Novartis save about $1 billion in operating costs by 2024 from a full restructuring.
Ji-Pu Viewpoint:
In order to maximize the interests of shareholders, pharmaceutical companies and medical devices have begun to split up their business divisions, such as GSK splitting up Haleon, GE splitting up GE healthcare, J&J splitting up Kenvue, Zimmer splitting up ZimVie and Metronic splitting up connected care, to strengthen sales, operating profit and cash flow for sustainable growth. care, in order to strengthen sales, operating profit and cash flow continued growth in the company's business investment and shareholders' rate of return, the integration of new business models and access to a new round of growth capital, compared with the large company business body operating model, the use of low-growth business divisions to form a focus on specific areas of the small and beautiful companies, and then through integration or mergers and acquisitions to create a new market value is also a good strategy.
Related news link: News
Novartis Confirms Sandoz Spin-Off Plan and Sets October 4 Completion Date |Biospace |
Sep W1|Big Health News Highlights: Life Sciences Giant Danaher to Buy Abcam for $5.7 Billion
Danaher Corporation, one of the world's largest suppliers of medical and diagnostic tools, has expanded its product and service portfolio with the acquisition of Abcam Plc, a manufacturer of protein/antibody and reagent consumables, in an all-cash transaction of $5.7 billion, including debt. The transaction will help Danaher to gain access to high-margin, recurring consumable revenues, and is expected to expand its full product line coverage.
Ji-Pu Viewpoint:
The Danaher Group has once again made a move for Abcam, a global provider of antibodies, reagents, biomarkers, and test kits for drug discovery, research, and diagnostics. This strong integration takes Danaher to new heights with the acquisitions of Aldevron, a manufacturer of mitochondrial DNA, mRNA, and recombinant proteins for vaccines, genetics, and cellular therapeutics, for $9.6 billion in 2021, and GE Biopharma, a biopharmaceutical solutions company with instrumentation, consumables, and software, for $21.4 billion in 2019. Aldevron, a producer of plasmid DNA, mRNA and recombinant proteins for vaccines, genetics and cellular therapies, and GE Biopharma, a biopharmaceutical solutions company with instruments, consumables and software, for $21.4 billion in 2019, rounding out the portfolio of more than 20 life sciences instrument, testing and consumables brands such as Pall, Beckman Coulter Life Sciences, SCIEX, Leica, Microsystems, Microsystems, Leica and Pall. Microsystems, Molecular Devices, Phenomenex and IDT, selling round lucre tools is still a good business in the biopharma gold rush.
Related news link: News
Life Sciences Company Danaher to Acquire Abcam for $5.7 Billion |reuters |
Aug W4|Big Health News Highlight: BTC kicks off with seven key recommendations from experts
Specific discussion points of the 2023 Biotechnology Industry Strategy Advisory Committee (BTC) include "Industry Infrastructure", "Benefit Systems", "Biomedical Data and Data Governance", "AI and Generative AI Technology Platforms", "Intelligent Healthcare, HIS, and Telemedicine", "CDMOs and Cellular Therapies", as well as "International Manufacturers' Coming to Taiwan and the International Market".
Ji-Pu Viewpoint:
Technological and scientific advances are accelerating the development of the biotech medical industry. In addition to the innovative applications of smart medicine, smart health, cellular therapy, or gene technology to meet medical needs, there are still many challenges in the implementation of healthcare laws and regulations, information security, or healthcare coverage, etc. From the perspective of strategic resources, the liberalization of healthcare laws and regulations, or adjustments is the first key step, along with the support of social healthcare resources and market capital, which is expected to drive the continuous upgrading of Taiwan's biotech medical industry. Taiwan's biotechnology and medical industry will continue to upgrade.
Related news link: News
Aug W3|Big Health News Highlight: ZimVie and Brainlab Deepen Collaboration on Spine Surgery Technology
ZimVie's Vital and Virage minimally invasive spinal immobilization systems, in conjunction with Brainlab's spine-specific software products, utilize AI, mixed reality (AR/VR), and other high tech tools to construct digital models of each patient's anatomy, which can help guide the surgeon through the entire procedure, as well as the hardware equipment, including robotic assistants and imaging.
Ji-Pu Viewpoint:
In recent years, medical material manufacturers have been spinning off their own businesses frequently. ZimVie is an independent new company focusing on spine and dentistry that will be spun off from Zimmer Biomet, a major manufacturer in the field of bone healthcare, in the spring of 2022, and this time the company announced its deepening cooperation with Brainlab, which emphasizes on Brainlab's AI+VR assisted surgical software system (Metacosmos Healthcare). Integration benefits, in addition to expanding the sales product line, through the integration benefits between implant consumables and surgical instruments and application software products, to provide a complete output value strategy for spine surgical products.
Related news link: News
ZimVie and Brainlab enhance spine surgery technology partnership|fiercebiotech |
Aug W2| Novo Nordisk Announces Results of Clinical Trial of Wegovy, a Weight Loss Drug, to Reduce Risk of Heart Disease in 20%
Danish pharmaceutical company Novo Nordisk (8/8) said that its weight-loss drug Wegovy can reduce the incidence of serious cardiovascular events, including stroke, in overweight or obese people with a history of heart disease by 20%, exceeding expectations. After the results came out, its stock soared more than 15%, becoming the highest market capitalization of European companies after LVMH boutique group.
Ji-Pu Viewpoint:
As obese patients are often associated with an increased risk of cardiovascular disease, but to date no drug has been approved to effectively control weight while reducing the risk of heart attack, stroke or cardiovascular death, the "miracle drug for weight loss," Wegovy, through a five-year large-scale clinical trial "SELECT," recruited a total of 17,600 people with confirmed cardiovascular disease, and overweight or obese, but no history of diabetes, the study results show that the drug can reduce the risk of severe cardiovascular disease up to 20%, approved for expanded use in cardiovascular disease indications will lead to an increase in the use of Wegovy will be about 25%. The results of the study showed that the drug can reduce the risk of serious cardiovascular disease up to 20%, and the approval of the expansion of the use of cardiovascular disease indications will lead to an increase in the utilization rate of Wegovy by about 25%.
Related news link: News
New Weight Loss Drug Wegovy Optimistic Data Fuels Innovation | The Wall Street Journal |
Wegovy reduces risk of cardiovascular disease, Novo Nordisk shares surge more than 17%|Financials |
Aug W1|Next-generation gene sequencing will be covered by health insurance next year, a major breakthrough in the precision medicine industry.
The Department of Health Insurance (DHI) of the Ministry of Health and Welfare (MOHW) has announced that next-generation gene sequencing (NGS) will be included in health insurance from 2024 onwards to assist in the precise use of drugs for cancer treatment. With the budget for new drugs/new technologies using health insurance, the Department of Health Insurance will carry out the relevant front-end supporting operations in the second half of this year, and it is expected to be finalized by the end of this year. As for the part of drugs that are selected for treatment after testing, the Health Insurance Agency will also accelerate the opening as much as possible and establish a "tentative payment strategy", in which NGS will make reference to the approach that "benefits must include the standardized sites before they are paid".
Ji-Pu Viewpoint:
Genetic testing is an important tool toward precision medicine, with the need for treatment, more and more cancer drugs need to correspond to the multi-site next-generation gene sequencing, in addition to multiple cancers synchronized detection of cancer early screening is also an important development trend, as well as disease, genetics, and clinical medical database development of the application of the construction of the NGS is expected to be incorporated into health care is expected to provide the living water of Taiwan's precision medicine industry, but due to the health care resources are limited, the principle of coverage is expected to be prioritized to cover the drug treatment "biomarker", such as drug coverage requirements have specified the scope of the relevant norms or licenses and take into account the cost-effectiveness of health care. However, due to limited health insurance resources, it is expected that the principle of coverage will be based on prioritizing the coverage of "biomarkers" involved in drug treatment, such as drug coverage requirements have been specified in the relevant specifications or the scope of application of the license and the comprehensive consideration of the cost-effectiveness of health insurance.
Related news link: News
Jul W4Using AI to Screen Drugs and Design Clinics, Taiwanese Companies Rush to Meet International Standards
Former U.S. President DonaldTrump was diagnosed during the epidemic, and was cured with antibody medicine through AI computation and analysis, setting off a huge business opportunity in the AI medicine market, and driving Taiwan-based pharmaceutical companies such as Pharmaceuticals China, Chi-King, ANH Biomedical, Molecular Intelligence Medicine, and Sage Advantage to lay out and join in the international race.
Ji-Pu Viewpoint:
Generative AI technology wave benefits continue to expand, the world crazy generative AI (Generative AI, generative artificial intelligence), because now AI can learn the language of various industries, generative AI so that the threshold of AI applications significantly lower and can be applied to all industries, which biotechnology and medical field is also an important target for the development of generative AI, through AI, generative AI in the drug discovery design (drug discovery new drug discovery), digital health clinics and smart medical applications play an important role in the development of Taiwan is not absent in the global generative AI development, Taiwan's AI computing power and digital health clinics and smart medical applications play an important role in the global generative AI development. With the support of AI, generative AI plays an important role in the development of drug discovery, digital health clinics, and smart medical applications. Taiwan is not absent from the global generative AI development, Taiwan's AI computing power and medical energy is an advantage, and it has built its own AI platform, and it is competing with the international large-scale companies in AI drug discovery and design by means of AI.
Related news link: News
Jul W3|Biotech company Recursion receives $50 million investment from Fidelity, stock price surges over 60% during trading session
Chip design company Phaidon NVIDIA announced that it will invest $50 million to accelerate artificial intelligence (AI) model training for drug discovery at biotech company Recursion Pharmaceuticals. Recursion and Phaidon have partnered to help solve one of the world's toughest challenges in drug discovery, utilizing NVIDIA DGX and NVIDIA AI software to create breakthrough work in biology and chemistry to accelerate the development of the world's largest biomolecule-generating AI models and accelerate drug discovery in biotechnology and pharmaceuticals.
Ji-Pu Viewpoint:
The main applications of AI drug discovery technologies in the drug discovery stage include target discovery, crystal shape prediction, and compound screening and optimization, Recursion's strength lies in AI image recognition technologies, such as image analysis of cancerous cells after drug treatment and fluorescent staining of biomarkers, which can be performed quickly and in large quantities for hit-compounds high-throughput parallel screening. Recursion is based on the BioHive-1 supercomputer with the latest NVIDIA GPUs, which is now combined with the NVIDIA DGX™ Cloud platform to extend the computing power and NVIDIA's new BioNeMo platform. BioNeMo platform for AI/ML training to develop breakthrough AI models with data from the biological and chemical fields, generative AI is expected to be a revolutionary tool for discovering new drugs and treatments.
Related news link: News
Jul W2|Pfizer's fat OEM order for Samsung Bio-Pharma is a big hit
Samsung Biologics announced on 7/4 that it had reached two foundry agreements with Pfizer for KRW 922.7 billion and KRW 254.3 billion in additional orders, totaling KRW 1.2 trillion (~USD 921 million) and Pfizer's orders for 2023 have totaled USD 1.08 billion, so far. Samsung BioTech's total contract value in hand has already exceeded the entire amount for the entire year of 2022.
Ji-Pu Viewpoint:
Samsung Bioepis' CDMO foundry manufacturing business opportunities are clearly trending, and new benefits are coming. In addition to winning Pfizer's nearly $900 million biosimilar foundry order, the company's customers also include Pfizer, Novartis AstraZeneca, Janssen, Eli Lilly, Merck (MSD), Roche, and GSK, and its subsidiaries Samsung Bioepis and Organon have obtained biosimilar licenses for Humira, a biosimilar to Humira. In addition, its subsidiaries Samsung Bioepis and Organon have also obtained a biosimilar license for Humira. Samsung Bioepis is capturing the hearts and minds of its customers' OEMs with its speed of execution and effective timeframe for obtaining regulatory approvals.
Related news link: News
Jul W1|US FDA's 6 New Drug Approval Decisions to Watch in Q3
By the end of September, the FDA will have held two consultation meetings and will have made key decisions on the release of six new drugs for depression, Alzheimer's disease, RSV, ocular retinal mapping atrophy, cardiomyopathy, and ALS.
- Sage and Biogen: New Drugs for Depression
- Eisai and Biogen: New Alzheimer's Disease Drugs
- Sanofi and AZ: New RSV Therapeutic Antibody Drugs
- Iveric Bio: A New Drug for Retinal Mapping Atrophy
- Alnylam: New drug for ATTR cardiomyopathy
- Brainstorm Cell Therapeutics: New Drugs for ALS
Ji-Pu Viewpoint:
Rare diseases, Alzheimer's disease and ophthalmology are the main focuses of recent new drug approvals. Rare diseases come from the results of the application of new technologies (new platforms), such as RNAi or cellular therapy; Alzheimer's disease comes from the common problem of human aging disability and the improvement of side effects, as well as the conditions of approval of the use of the drug and health insurance coverage policy; ophthalmology comes from the new function of the complementary C3/C5 proteins, which change the past of the retina to map atrophy without effective treatment options. In addition, the acquisition of new drugs for rare diseases and ophthalmology is also an important area for international pharmaceutical companies to advance their business.
Related news link: News
5 FDA decisions to watch in Q3 | Biopharmadive |
Jun W4|Medtronic Contracts to Distribute Allurion Weight Loss Surgery Equipment and AI Device
Medtronic has contracted to distribute Allurion's swallowable intragastric balloon, which holds the stomach in place for approximately four months before naturally contracting and leaving the body through the digestive system. During this time, patients can monitor weight loss progress and guide behavioral changes using Allurion's Smart Weigher, Healthy Smart Watch and Weight Management App, as well as the Iris AI Virtual Care Platform. In addition Allurion is preparing to achieve its go-to-market goal through a merger with special purpose acquisition SPAC in the coming weeks.
Ji-Pu Viewpoint:
The business layout of medical equipment manufacturers is developing towards intelligent total solution solutions. Take Allurion's weight loss equipment layout as an example, it includes smart weight machine, health smart watch, weight management APP, and Iris AI virtual care platform (AI algorithms to predict the effect of patients' weight loss programs), which not only helps doctors to track the progress of patients' treatment effect, but also serves as a tool for medication or nutritional assistance for weight loss. In addition to helping doctors to track the progress of patients' treatment, the Iris AI can also be used as a tool to assist weight loss through medication or nutrition.
Related news link: News
Jun W3|Samsung BioCDMO to double output by 2032, focus on ADCs and CGTs
Samsung Biologics' CDMO plans to invest US$5.4 billion in four new plants in the future to increase its capacity to 1.3 million liters by 2032 to further widen the gap with global competitors. The company's current annual production capacity is 604,000 liters, and the four new plants will create 720,000 liters of capacity in 10 years, and is optimistic about the demand for CDMO services for antibody drug complexes (ADCs) and cell and gene therapy (CGT). In addition, the company is optimistic about the demand for CDMO services for Antibody Drug Complex (ADC) and Cell and Gene Therapy (CGT).
Ji-Pu Viewpoint:
As global competition for new drug development heats up, the mushrooming development of various new drug R&D fields has also led to the expansion of CDMO supply chain capacity. The three key elements for CDMO development are high-quality technology, sufficient capacity, and the ability to expand customers, Samsung BioTech has recently indicated that it has already secured 13 customers among the world's top 20 pharmaceutical companies, which is an announcement of the 10-year development plan, said Samsung BioTech. Samsung BioTech is optimistic about the foundry manufacturing with biologics as the core, and even more optimistic about the new drug development opportunities of ADC and CGT.
Related news link: News
Samsung Biologics to double production by 2032; eyes ADCs, M&As |The Korea Economic Daily |
Samsung Biologics accelerates timeline of new fifth plant to be operational by April 2025 |CISION |
Jun W2|FDA considers temporary importation of anti-cancer drugs from unapproved companies to ease U.S. supply shortages
CNN, CNBC and Wall Street Journal report that there is an acute shortage of cancer drugs in the United States, affecting hundreds of thousands of patients. The U.S. Food and Drug Administration (FDA) recently announced that it has approved and imported the Chinese-made chemotherapy drug cisplatin in an attempt to alleviate the shortage. In fact, not only anti-cancer drugs, the United States has been experiencing a severe shortage of drugs, and many common drugs are in short supply. According to the FDA, there are currently shortages of more than 100 drugs in the United States.
Ji-Pu Viewpoint:
From the perspective of the API supply chain, nearly 80% of active pharmaceutical ingredients in the U.S. are produced outside the U.S., mostly from China or India. There is a shortage of at least 14 anticancer drugs in the U.S., with carboplatin and cisplatin being the main ones. The shortage of raw materials for these drugs originated from the temporary closure of Intas Pharmaceuticals in India due to quality problems, which resulted in the inability to manufacture downstream pharmaceuticals such as Fresenius Kabi, Hikma Pharmaceuticals, and Teva. In addition, most of the precious metal platinum for platinum drugs comes from South Africa and Russia, where there is a shortage of electricity and a war between Russia and Ukraine, and the risk of uncertainty in the supply chain of raw materials has also contributed to the worsening shortage of drugs recently.
Related news link: News
Jun W1|Novo Nordisk (NVO.US) and Pfizer (PFE.US) say their oral weight-loss drugs are as effective as injections
Novo Nordisk (NVO.US) and Pfizer (PFE.US) have released data showing that their oral weight-loss drugs are about as effective as injectable drugs. Novo Nordisk said that in one case, its oral Semaglutide helped overweight or obese adults lose weight in a way that was comparable to Wegovy® (Semaglutide) injections. In another, data showed that Pfizer's oral weight-loss drug danuglipron was as effective as Novo Nordisk's Ozempic® (Semaglutide) injection.
Ji-Pu Viewpoint:
Since its launch in the U.S., NOVO's Wegovy weight-loss drug has transformed the weight-loss market, attracting the attention of patients, investors and celebrities around the world. The huge demand for weight loss treatments will drive sales of up to 10 competing products, which are estimated to generate annual sales of up to US$100 billion over 10 years. In addition, the introduction of oral dosage forms, which eliminate painful injections, will change the landscape of the weight loss and diabetes markets.
Related news link: News
May W4|Taikang Biotech's breast cancer biosimilar is approved by the Ministry of Health and Welfare to be marketed in Taiwan.
Tecan Biotech (6589) (16th) announced that its self-developed breast cancer biosimilar EG12014 "EIRGASUN Cold Crystalline Injectable 150 mg" has officially received a letter of approval from the Ministry of Health and Welfare's Food and Drug Administration (FDA), and that EIRGASUN has been granted the official Taiwan Drug Certificate (TDC). EIRGASUN will submit the application for health insurance coverage to the Department of Health Insurance in the shortest possible time, and we look forward to the second half of the year when we can start to attack the original drug Herceptin.®(trastuzumab) with an annual market opportunity of NT$2.388 billion.
Ji-Pu Viewpoint:
2019/12 AZ Co-develops Enhertu with Daiichi Sankyo® (Tecan's trastuzumab deruxtecan ADC, with sales of more than US$1 billion by 2022, has created a sensation in the market and increased interest in the extended application of HER2 drugs. Tecan has reached the milestone of trastuzumab biosimilar development, and continues to advance the development of a series of HER2-positive breast cancer product portfolio, and is committed to expanding to the development of new ADC drugs and dual-targeted therapeutics, which is worth looking forward to in terms of the future development layout in terms of topic and development strategy.
Related news link: News
Tecan Biotech's "Yikangpin" Obtains Taiwan Pharmaceutical Certificate| Business Times
May W3|ADC's new drug development is in full swing, and we are seeing a new layout.
Japanese pharmaceutical company Eisai and Chinese start-up Bliss Bio have signed a $2 billion co-development agreement for BB-1701 antibody drug coupling (ADC drug) oncology drug.
Ji-Pu Viewpoint:
ADC drugs have become a must for international pharmaceutical companies. The ADC market has been growing steadily over the past five years, with sales approaching $7 billion by 2022. With the acquisition of Seagon by Pfizer/Wyeth, various teams have emerged, including Genetech/Roche, AZ (Daiichi Sankyo/China's LaNova), Gliead (Immunomedics), and GSK (Mersana Therapeutics), among which Eisai (Bliss Bio) is also in the game, Gliead (Immunomedics) and GSK (Mersana Therapeutics), with Eisai (Bliss Bio) also joining the development wave.
Related news link: News
Nippon Eisai signs $2B oncology deal with Chinese startup Bliss Biopharma| biospectrumasia|
May W2|2023 National Biotechnology Park Demo Day Highlights
The National Biotechnology Research Park Demo Day debuted this year, focusing on the dual axes of "industry cross-domain" and "resource linkage", gathering BIO-ICT cross-domain experts in a series of forums to share the niches of cross-domain integration, and to stimulate the possibility of cooperation and business opportunity exploration, as Taiwan is moving towards the transformation of digital healthcare to promote the re-upgrading stage of the precision health industry.
Ji-Pu Viewpoint:
Three key observations from the conference: (1) The precision health industry is data-centric, driving the integration of genetics, AI, big data, and personalized healthcare benefits, and continues to ferment in the precision healthcare industry ecosystem, but the business model is still groping for breath; (2) Taiwan's advantages in developing the precision health industry, as shown in Table 1, include medical big data, Taiwan's key ICT technologies, and Taiwan's expert talent; (3) The international biotech industry is pursuing hot areas, focusing on oncology (highly competitive), rare diseases (highly competitive), obesity (rising), and RNA technology (rising), benefiting from a number of databases. The pharmaceutical industry is pursuing hot areas focusing on oncology (highly competitive), rare diseases (highly competitive), obesity (on the rise), and RNA technology (on the rise), benefiting from a number of dataset announcements, which will boost the development of neuroscience, next-generation immunotherapies, and autoimmune drugs.
Table 1: Taiwan's Advantages in Developing the Precision Health Industry
Source: Organized by Ji-Pu Industrial Trend Research Institute.
Related news link: News
May W1|US FDA Approves First Oral Microbiota Therapy Medication
The US FDA has approved Seres Therapeutics Inc (MCRB.O) SER-109 (trade name: Vowst), an oral microbiota therapy, for the prevention of recurrent Clostridium difficile (C. diff) infection (rCDI) after antibiotic treatment, which is usually caused by prolonged use of antibiotics that destroy beneficial colon bacteria, leading to potentially fatal diarrhea and colitis. This can lead to potentially fatal diarrhea and inflammation of the colon.
Ji-Pu Viewpoint:
In recent years, microbiome has been one of the hotspots for new drug development. It is known that human gut microflora is an indicator of mental health, neurodegenerative diseases, gastrointestinal disorders, and cancers, etc. With the technological breakthroughs of gene sequencing analysis to monitor the changes of the intestinal flora and in vitro culturing, the microbiome has become more comprehensive in the development of new drugs and clinical applications. This first FDA approval for oral fecal microbiome therapy symbolizes an important milestone in the development of microbiome therapeutics.
Related news link: News
FDA approves Seres Therapeutics' pill for deadly Clostridium difficile infection|Reuters|
FDA approves first oral microbiome treatment Vowst: Will Nestle help Seres to market? |Fiercepharma
Apr W3|Expanding Immunotherapy Landscape: Merck Sharp & Dohme's $10.8 Billion Takeover of a Peer Group
U.S. biopharmaceutical giant Merck (Merck, or Merck Sharp & Dohme) will buy Prometheus Bioscience for $10.8 billion, a move that not only compensates for the impact on revenue of its star drug Keytruda, which will lose its exclusive rights to sell it in 2028, but also sets the stage for a new layout of the immunotherapy landscape.
Ji-Pu Viewpoint:
MSD's acquisition will provide Prometheus with a drug candidate for the treatment of Inflammatory bowel disease (IBD), and is expected to win the first in class new target TL1A mAb and expand its business opportunities in the autoimmune field. Autoimmune diseases and immunotherapy have always been the areas that international pharmaceutical companies must fight for after the oncology drug market. It is not difficult to see from the topic selection strategy of international pharmaceutical companies that it is still worthwhile to fight for drugs in the areas of atopic dermatitis (AD), ulcerative colitis (UC), or Crohn's disease (CD). It is still worthwhile to try.
Related news link: News
Mergers and Acquisitions: $10.8 Billion |Economic Daily News
Apr W2|Pear Therapeutics, a pioneer developer of digital therapies, declared bankruptcy.
Pear Therapeutics confirms that while the company's digital therapy products could improve the company's internal financial metrics and patient health, the company has been slow to secure payer coverage.Pear Therapeutics has launched a number of prescription digital therapeutics (PDT) but has failed to deliver results and announced that it will lay off more than 90% employees while it seeks a buyer. Pear Therapeutics has launched a number of prescription digital therapeutics (PDTs) that have failed to bear fruit, and has announced that it will be laying off employees above the 90% level as it seeks a buyer.
Ji-Pu Viewpoint:
The collapse of Pear Therapeutics, the world's leading digital therapy startup, reflects the current dilemma of U.S. biotech startups that are struggling to raise capital. Although the prescription digital therapy industry has overcome the US FDA approval hurdles and more and more products have been certified by the US FDA, whether or not customers can pay the high cost of using prescription digital therapy products is still the main problem facing prescription digital therapy products at this stage. In addition, the US banking risks and recession concerns are also factors contributing to the difficulties in raising funds for biomedical start-ups at this stage, the poor prospects for IPOs, the scarcity of mergers and acquisitions, and layoffs. In addition, banking risks and economic downturn concerns in the U.S. are also contributing to the difficulties in raising capital for biomedical start-ups, poor IPO prospects, few mergers and acquisitions, and layoffs.
Table 1: Pear Therapeutics Approved Digital Therapeutic Products
Source: Organized by Ji-Pu Industrial Trend Research Institute.
Related news link: News
Pear Therapeutics applies for bankruptcy protection|mobihealthnews|
Apr W1|Emergency Authorization EUA will go into history U.S. FDA proposes emergency authorization termination transition period
The US FDA announced on March 24th that the Emergency Use Authorization (EUA) for COVID-19 will be terminated on May 11th, and that medical technology companies that have received an EUA in the past will need to prepare a transition plan as soon as possible. Companies that continue to sell medical devices under EUA will need to submit a marketing submission to the US FDA prior to the termination date of EUA. If accepted by the US FDA, the company may continue to sell the device during the review period, while those that do not submit such a submission to the US FDA will have to stop selling the device according to the deadline.
Ji-Pu Viewpoint:
As the epidemic recedes and countries unseal and open up, the corresponding epidemic demand for emergency use authorization or partial exemption from the permit to market the medical materials will also return to the regular management of medical materials, this time the US FDA proposed the termination of emergency authorization and the 180-day transition period, in line with the industry's expectations is not unexpected, the scope of the medical materials covered by the current equipment such as in-vitro diagnostic, blood purification equipment equipment, cameras, ventilators, oxygen generators, Yekke film or remote digital pathology equipment, etc. Although these devices will not be subject to the impact of short-term inventory and supply chain reserve market shortages. Although these devices will not be impacted by short-term inventory and supply chain stocking shortages, they may still have an impact on in vitro diagnostic products, which are subject to a relatively large number of reviews, due to the speed of review and the volume of reviews being crowded out.
Related news link: News
Transitioning US COVID-19 EUA Devices after EUA Termination|Qservegroup|
Taiwan's regenerative medicine to be included in commercial insurance and health insurance
The "Regenerative Medicine Bill" and the "Regenerative Medical Agents Bill" (the "Regenerative Medicine Bill") will be reviewed on the 20th. Regenerative medicine is expensive and is tantamount to "healthcare for the rich," so the Ministry of Health is planning to ask the Financial Supervisory Commission (FSC) and the insurance industry to study how to help through commercial insurance or health insurance.
Ji-Pu Viewpoint:
Regenerative medicine utilizes cells to produce medicines, or cells to produce body tissues to cure diseases, and moves from the dual tracks of medical technology and new drug development to the application of clinical diseases. Since the opening of Taiwan's regenerative medicine special regulation, it has gradually opened up the development capital (industry fundraising/tax incentives), temporary license permit (collateral permit) and charging mechanism, and has converged with the healthcare service market. Recently, in order to reduce the burden of healthcare on the public, it has been proposed to be included in the commercial insurance and healthcare insurance to share the cost, which is expected to promote the regenerative medicine treatment services to be more friendly to the public, and to drive the accelerated development of the regenerative medicine.
Table 1: Regulations and Policies on Regenerative Medicine
Source: Organized by Ji-Pu Industrial Trend Research Institute.
Related news link: News
Regenerative medicine to be included in business insurance and health insurance|Business Times
SVB Crisis and Opportunity for Biotech New Ventures in a Banking Storm
Fortune and CNBC report that SVB, which closed on March 10, was a very important financing bank for life sciences and health care start-ups. By the end of 2022, VC-backed health care start-ups accounted for 121 TP3T of SVB's $173 billion in deposits and 361 TP3T of its $168 billion in off-balance-sheet financing.
Ji-Pu Viewpoint:
Silicon Valley Bank (SVB) has been betting heavily on biotech innovation in recent years, and is a very important financing bank for life sciences and healthcare start-ups. Nearly half of the U.S. technology VCs and biotech VCs have established cooperative relationships with SVB, and SVB's closure will affect the capital chain of U.S. small and medium-sized biotech companies, and the valuation of small and medium-sized biotech companies will decline, and the fundraising situation will worsen. For pharmaceutical companies and investment organizations with capital in hand, it is expected that they will enter the market at a low price or acquire small and medium-sized biotech companies with technological value. This is the worst time to raise capital and the best time to invest.
Related news link: News
U.S. biotech ETFs dive as SVB collapses, even as biotech startups suffer|MoneyDJ|
FDA approves Intellia's in vitro gene editing for U.S. clinical trial
The U.S. FDA has approved gene editing therapy company Intellia Therapeutics to conduct a human trial of an experimental CRISPR drug, NTLA-2002, for the treatment of hereditary angioedema, which has been studied in the Netherlands, the U.K., and New Zealand up to this point, and the U.S. FDA is allowing U.S. patients to participate in the Phase II trial, which indicates that The FDA's decision to allow U.S. patients to participate in the Phase II trial suggests that the U.S. FDA may be more comfortable with in vivo gene-editing drugs.
Ji-Pu Viewpoint:
With the success of mRNA vaccines, advances in liposomal nanoparticle (LNP) delivery technology, combined with gene editing technology represented by CRISPR-Cas9, is accelerating the future of gene therapy for the development of rare diseases.Intellia Therapeutics announced the U.S. FDA approval of its investigational intracorporeal gene editing CRISPR therapy, NTLA-2002, for the treatment of hereditary angioedema (HAE). Intellia Therapeutics announced that the U.S. FDA has approved the application of NTLA-2002, an investigational new drug (IND), for the treatment of hereditary angioedema (HAE), which is a highly efficient in vivo gene editing therapy delivered through LNP injection, opening up new avenues for the treatment of many hereditary diseases and expanding the scope of application of gene editing therapies. The FDA's approval of Intellia's in vivo gene editing drug for clinical trials in the U.S. not only indicates that the U.S. FDA may be more comfortable with in vivo gene editing drugs, but also symbolizes a new breakthrough in the potential application of in vivo gene editing drug technology.
Related news link: News
Gene editing 'in vivo'! Intellia to conduct in vivo trials in the U.S.A. for rare diseases
Drug shortage is on the rise! The Ministry of Health and Welfare listed 6 major reasons for the shortage of drugs and 15 types of drug items at a time.
The tide of drug shortages is spreading! Statistics of the most commonly used and short of drugs in the community pharmacy in Taiwan, a total of more than 10 kinds of drugs, can be broadly divided into five categories, including antibiotics, three high drugs, gastrointestinal drugs, epilepsy drugs and allergy-related drugs hospital shortage of drugs, although less than a complete out of stock, but the supply is about 70 ~ 80%, "respiratory diseases" and "anticoagulant drugs" shortage.
Ji-Pu Viewpoint:
Shortage of drugs storm blowing into Taiwan, in fact, the epidemic, war and economic structure, highlighting the globalization of the pharmaceutical industry chain structure problems, with the unstable global supply of raw materials (APIs), affecting the whole body, Taiwan can not be avoided, in the past decade, the production of raw materials needed for drugs (APIs) shifted to the much lower cost of India and China and over-concentration, resulting in economic factors, the API supply chain As a result, the supply chain of APIs is inflexible and insufficient due to economic factors, and shortages of antibiotics will become the norm. In addition, a large number of elderly, chronically ill and infectious disease populations around the world require antibiotic treatment. After the epidemic, countries were unsealed (no need to wear masks on public occasions), and crowds began to gather, which led to a huge increase in the number of people with respiratory infections, which also led to a huge increase in the demand for antibiotics for respiratory infections, and a shortage of antibiotics has continued to spread due to the mutual squeeze between supply and demand. Currently, Europe, the United States, Canada, and Australia have all reported antibiotic shortages. Therefore, by acquiring API manufacturers first, we are expected to seize the business opportunities in the drug shortage market and turn the market crisis into a turning point.
Related news link: News
Strike Again! GE Healthcare Acquires AI Caption Health's Perfect Ultrasound Layout
GE HealthCare 2/9 announced the signing of an agreement to acquire AI-assisted ultrasound vendor Caption Health. With Caption Health AI-assisted, ultrasound exams can be easier and faster, enabling more healthcare professionals to perform basic ultrasound cardiograms.
Ji-Pu Viewpoint:
Through a series of acquisitions over the years, GE Healthcare has become a leader in the ultrasound field. Its products cover the high, mid and low end markets, and it is a leader in obstetrics and gynecology, radiology, and cardiac applications, and has expanded from diagnostic to surgical visualization. The acquisition of AI-assisted ultrasound Caption Health will not only add to GE Healthcare's ultrasound product portfolio, but will also expand the scope of ultrasound use. The acquisition of AI-assisted ultrasound Caption Health not only increases GE's medical ultrasound product portfolio and expands the scope of ultrasound usage to AI-assisted cardiac ultrasound to measure heart failure (ejection fraction), which will help to expand the number of healthcare professionals using the tool in different care environments under the guidance of AI-assisted ultrasound, but also anticipates the trend of medical device manufacturers aiming at the competitive edge of AI-assisted systems in the future, prolonging the market life cycle of their products, expanding the threshold of entry into the market, and making the combination of medical devices and AI a standard feature in the future. AI will become a standard feature in future medical devices. As of October 2022, GE Healthcare is the company with the most US FDA-approved AI medical devices, with about 42 devices, most of which are used in radiology.
Table I,GEMedical Acquisition of Ultrasound Business
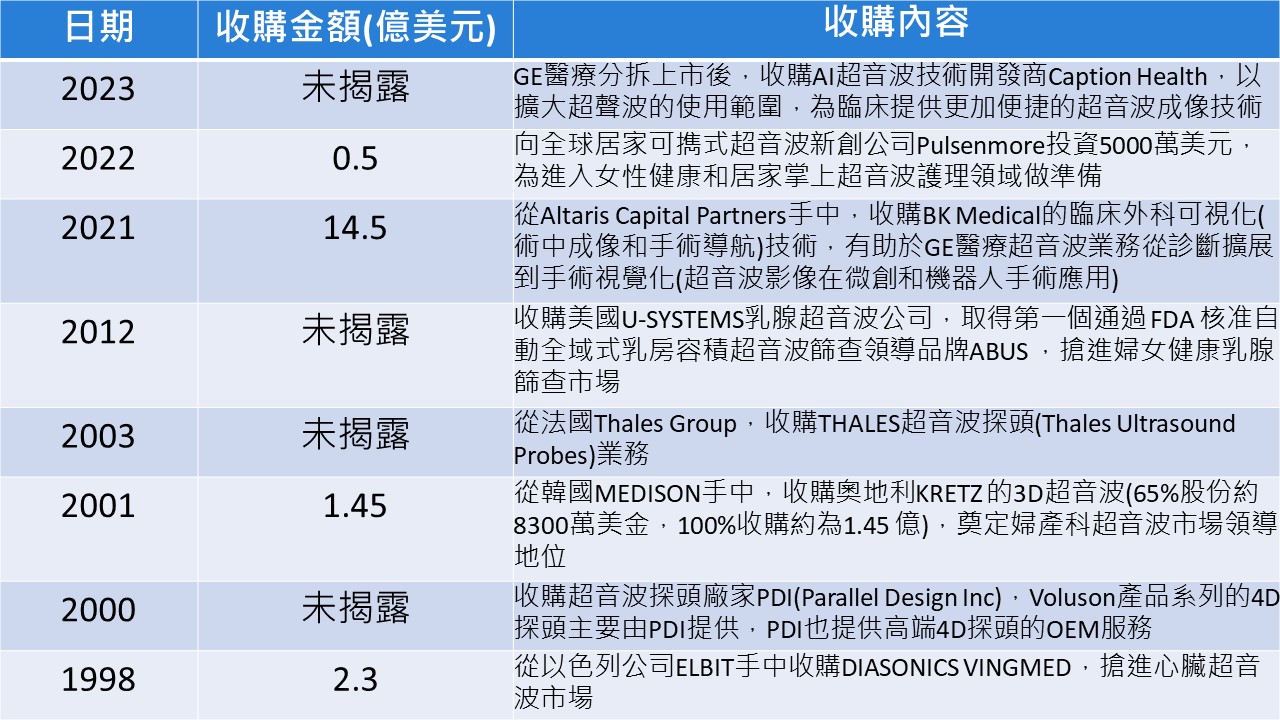
Source: Organized by Ji-Pu Industrial Trend Research Institute.
Related news link: News
GE HealthCare Acquires Caption Health, Expands Ultrasound Business with FDA-Approved AI Assist |WSJ|
Abbott Acquires CSI for $890 Million, Funding and Growth Momentum to Be Seen
2/8 Abbott agreed to acquire Cardiovascular Systems, Inc. (CSI) for approximately $890 million, a deal that will expand Abbott's portfolio of minimally invasive products for peripheral cardiovascular and coronary artery disease.
CSI's atherosclerotomy (atherectomy) device can polish away plaque buildup in the arteries. Minimally invasive treatments can help with balloon angioplasty (balloon angioplasty) or restoring blood flow with stents. The deal should help boost Abbott's competitive position in the sluggish vascular market.
Ji-Pu Viewpoint:
Medical devices is Abbott's division with the greatest potential. Abbott's leading position in the field of cardiac stents comes from its acquisition of St. Jude Medical in 2016, where it has taken the lead in both the cardiac stent and pacemaker fields, and has successfully occupied the cardiovascular field, which is the second largest market for medical devices with a market size of hundreds of billions of US dollars. In addition to expanding the product line portfolio, creating sales growth momentum, and acquiring key technologies, this renewed effort is also related to the strong financial momentum of Abbott's new coronary screening business during the epidemic, which is worth paying attention to the new round of capital acquisitions after the wave of the epidemic.
Table 1: Abbott's Cardiovascular Acquisitions
Source: Organized by Ji-Pu Industrial Trend Research Institute.
Related news link: News
Abbott to Acquire Cardiovascular Systems, Inc. |prnewswire | The New York Times
New Crown Drugs Sales Stronger Than Expected Merck Results
Merck & Co. of America (MRK-US) reported on 2/2 stronger sales of its new crown antiviral Lagevrio® (molnupiravir) in Asia and higher-than-expected 4Q2022 earnings.
Lagevrio® (molnupiravir) sales of US$825 million in the quarter were well above analysts' estimates by a factor of two at US$358 million. Sales of Lagevrio® (molnupiravir) in China were unaffected in the fourth quarter, primarily due to the wave of outbreaks in Asia in the fourth quarter that was able to drive sales of the drug, particularly in Japan, South Korea, and the rest of the Asia-Pacific region, where the drug was not approved for use until December 30th. The company forecasts molnupiravir sales of just under $1 billion in 2023, with most of that coming from Asian markets including China.
Ji-Pu Viewpoint:
COVID-19 new crown epidemic treatment drugs focus on three parts are a. prevention of viral infection, b. treatment of symptoms after viral infection, c. treatment of viral infection after the emergence of related complications of the treatment, with the epidemic slowed down, a number of large international pharmaceutical companies to reduce the company's new crown-related drug market sales estimates, such as the United States, Merck's new crown of the oral drug Molnupiravir (Molnupiravir) sales will fall sharply from $5.68 billion in 2022 to about $1 billion in 2023; Pfizer (PFE-US) reported its antiviral drug Paxlovid in 2022 from $5.68 billion to about $1 billion. For example, Merck's oral drug Molnupiravir will see its sales plummet from $5.68 billion in 2022 to about $1 billion in 2023; Pfizer (PFE-US) reported sales of its antiviral Paxlovid of about $18.9 billion in 2022, and expects sales of about $8 billion in 2023; and Pfizer (PFE-US) is forecasting sales of about $8 billion in 2023. However, the new crown of the epidemic fluctuates, with the lifting of the ban on foreign countries and other disruptive factors will affect the future amount of drug sales and corporate profits changes, the new crown of the influenza, the corresponding alleviation of symptoms of viral infections after the sales of drugs demand can still be expected.
Table I,Types of medications used for new crown therapy(USFDAApproved drugs)
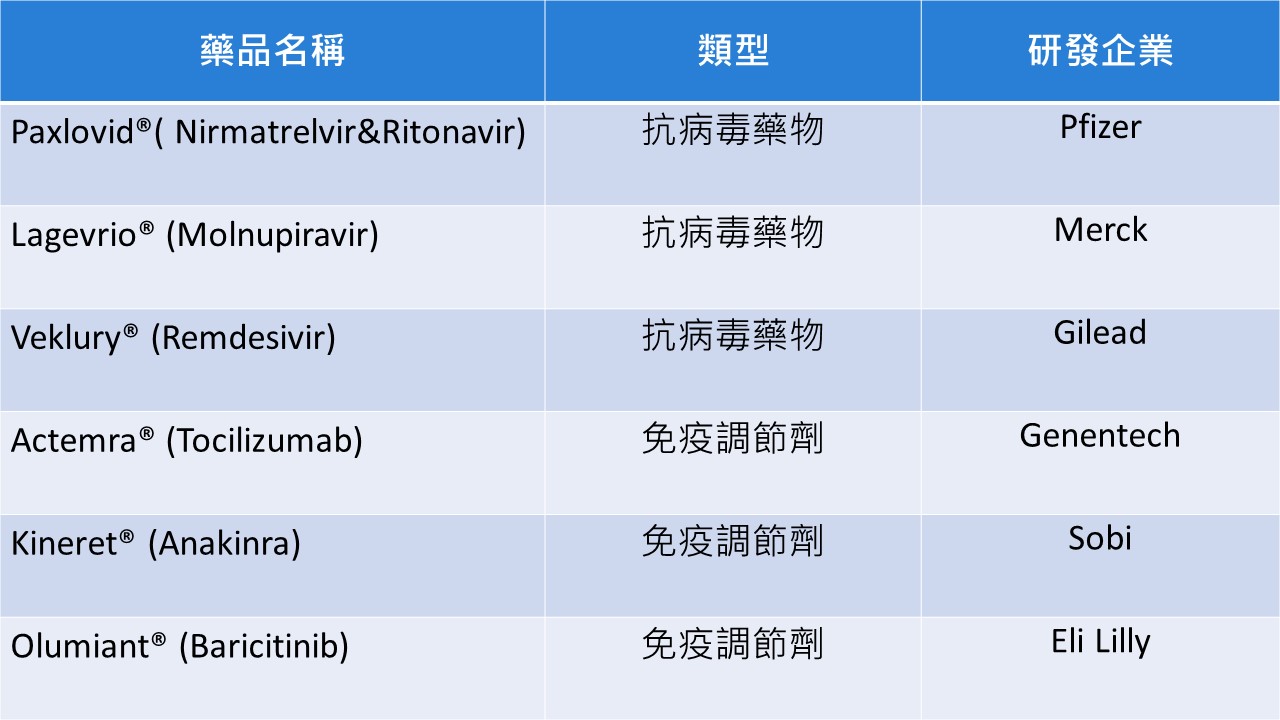
Source: Ji-Pu Industrial Trend Research Institute
Related news link: News
Amazon's New Service Offers Prescription Drug Subscription Delivery to Prime Members
Amazon (AMZN-US) 1/24 announced a new prescription offer for U.S. Prime members, hoping to increase subscriptions and attract users to its pharmacy services.
RxPass' add-on service will allow Prime members to get the amount of medication they need from a list of 50 generic drugs to treat more than 80 common chronic conditions, such as high blood pressure, anxiety, and diabetes. The service costs $5 per person per month with free home delivery.
Ji-Pu Viewpoint:
Amazon continues to expand its healthcare business in the face of the continued bullishness of U.S. tech companies such as Apple, Microsoft and Alphabet on the digital healthcare market, as well as U.S. pharmacy chains Walgreens and CVS Health, and even the retailer Walmart, which sees primary care as a business model for diversifying revenue sources and increasing revenues. Amazon has recently launched another experimental service, the prescription drug subscription and delivery service for Prime members, which will promote the digitization of primary care services and integrate its healthcare business resources more efficiently, just as CES2023 observed the telemedicine and virtual clinic services in the U.S., the benefits of which are worth observing in terms of implementation and popularization. The benefits of implementation and popularization are worth observing.
Table 1: Amazon Healthcare Layout
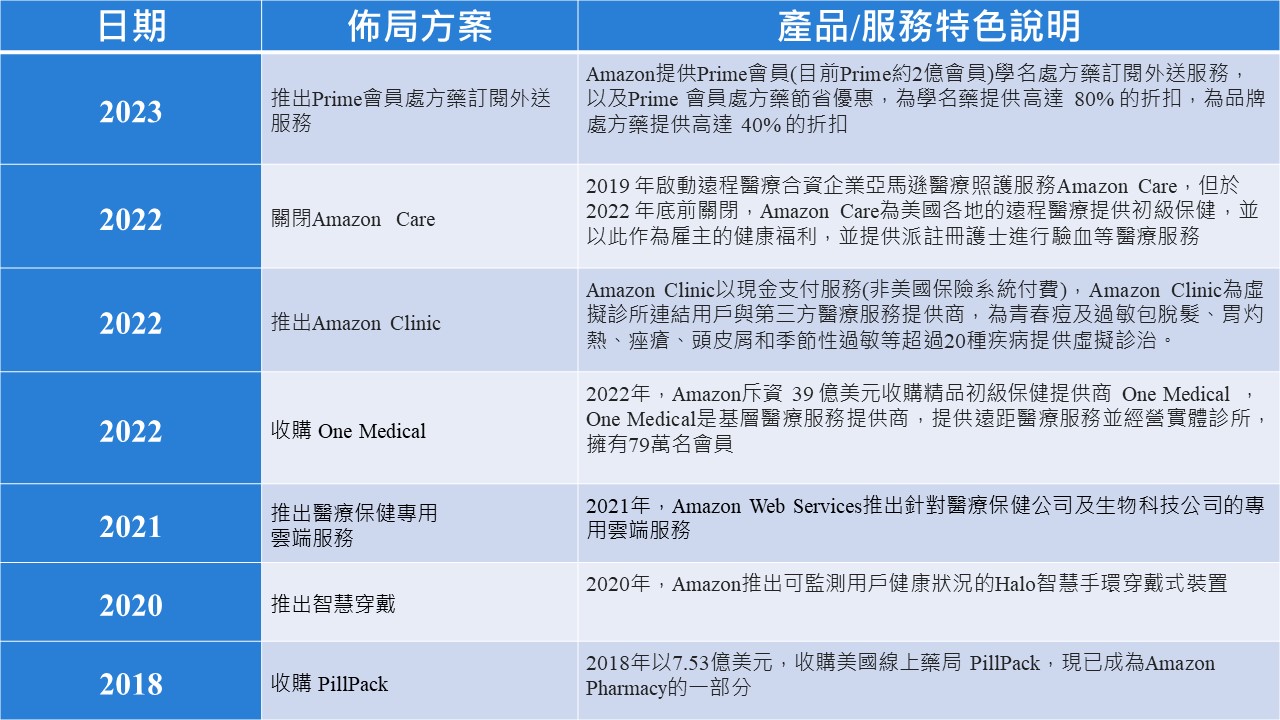
Source: Ji-Pu Industrial Trend Research Institute
Related news link: News
Amazon offers Prime members prescription drug subscription and delivery service|MaxOffice|MaxOffice
Telemedicine is poised to take off with the loosening of the regulations of the "Regulations on Telecommunication Clinic Treatment"?
The new crown pneumonia has led to the development of video clinic, the Ministry of Health and Welfare completed the draft amendment to the "Communication Clinic Treatment Regulations" preview, will be applicable to the expansion of the target population, including chronic patients, hospice patients, mobility impaired, infectious diseases and disaster victims, expatriates are applicable. The preview period of the draft expired at the end of January, and it will go into effect in February at the earliest.
Viewpoint: The relaxation of the regulations of the "Regulations on Telemedicine" will accelerate the expansion of the scope of telemedicine, the decentralization of hospitals, and the development of the medical care model towards multi-video diagnosis and treatment, which is expected to open up new business opportunities for the medical technology industry, but the actual implementation of telemedicine still needs to consider the adjustment of a number of laws and regulations, and in addition, with the unsealing of the epidemic, the change of the people's habits of medical care will be an In addition, after the epidemic is lifted, the change of Chinese people's medical consultation habits will also be an important observation indicator.
Table 1: Telemedicine-related Regulations and Practitioners
Source: Ji-Pu Industrial Trend Research Institute
Related news link: News
Five new categories of video therapy added for chronic, hospice patients|Chronicle News
Five new categories of video therapy added for chronic, hospice patients|Times-News
CES 2023 Digital Health Trend Highlights
CES 2023 digital health from mobile health, telemedicine, medical information, remote monitoring, nursing care, personalized health care to beauty technology! Three highlights: future care, women's health technology and care technology for the elderly, specific connotation for the future of care care big changes, home care into the mainstream, medical solutions to the home; women's technology hidden hundreds of billions of business opportunities "women's health technology" is in the way; elderly care and care pressure has been increasing, digital health technology connecting the safety net of home life is tamping, the exhibition highlights of the product: AI stethoscopes, the most lightweight electrocardiography equipment (atrial fibrillation), conductive-free health care technology, and the most important products. AI stethoscope, the lightest ECG device (atrial fibrillation alert), wire-free pacemaker and urine health detector attracted a lot of attention at the show.
Table 1, CES 2023 Eye-catching Products
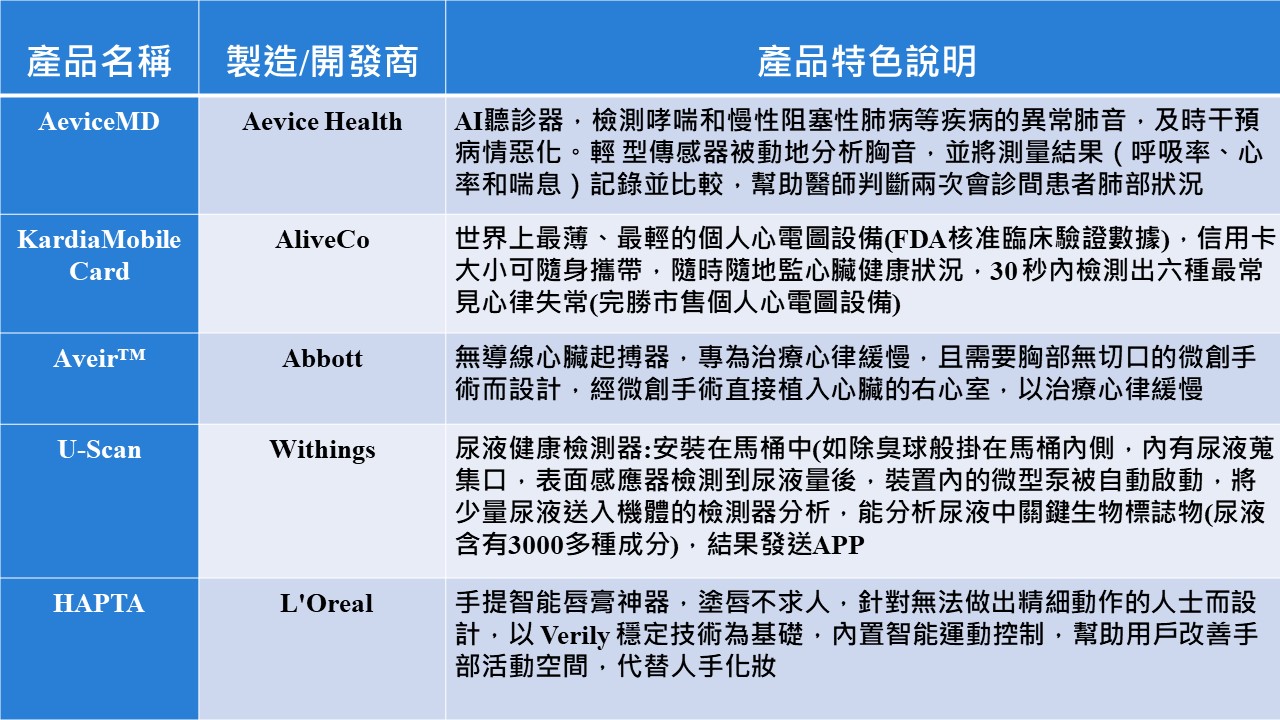
Source: Ji-Pu Industrial Trend Research Institute
Related news link: News
CES highlights summary|Business Times|The first post-epidemic show with new product launches
CES 2023] This tool hangs on the toilet with an iPhone and can detect your urine|News from Nutella
Cash-rich M&A resumes for several large pharmaceutical companies, benefiting from COVID-19's best-selling vaccine, growing sales of innovative branded drugs, and high-value divestitures!
3 Largest Acquisitions in the Pharmaceutical Industry in 2022
- Amgen Acquires Rare Disease Specialty Drug Maker Horizon Therapeutics for $27.8 Billion
- Pfizer Acquires Dual-Action Migraine NURTEC ODT Firm Biohaven Pharma for $11.6 Billion
- Pfizer Acquires Sickle Cell Disease for US$5.4 BillionOxbrytaGlobal Blood Therapeutics.
It is worth noting that Amgen acquired ChemoCentryx and Tavneos, a rare vasculitis autoimmune drug, for US$3.7 billion in October 2022. The acquisition of Horizon Therapeutics, a specialty pharmaceutical company for rare diseases, demonstrates Amgen's determination to establish a presence in the rare disease field. The acquisition of Horizon Therapeutics, a specialty pharmaceutical company for rare diseases, demonstrates Amgen's determination to expand its business in the field of rare diseases. From the selection of topics by international pharmaceutical companies, the field of rare diseases has been one of the most important choices for international pharmaceutical companies to expand their business in the near future.
Table 1: Information on Acquisition Transactions of International Major Manufacturers in 2022
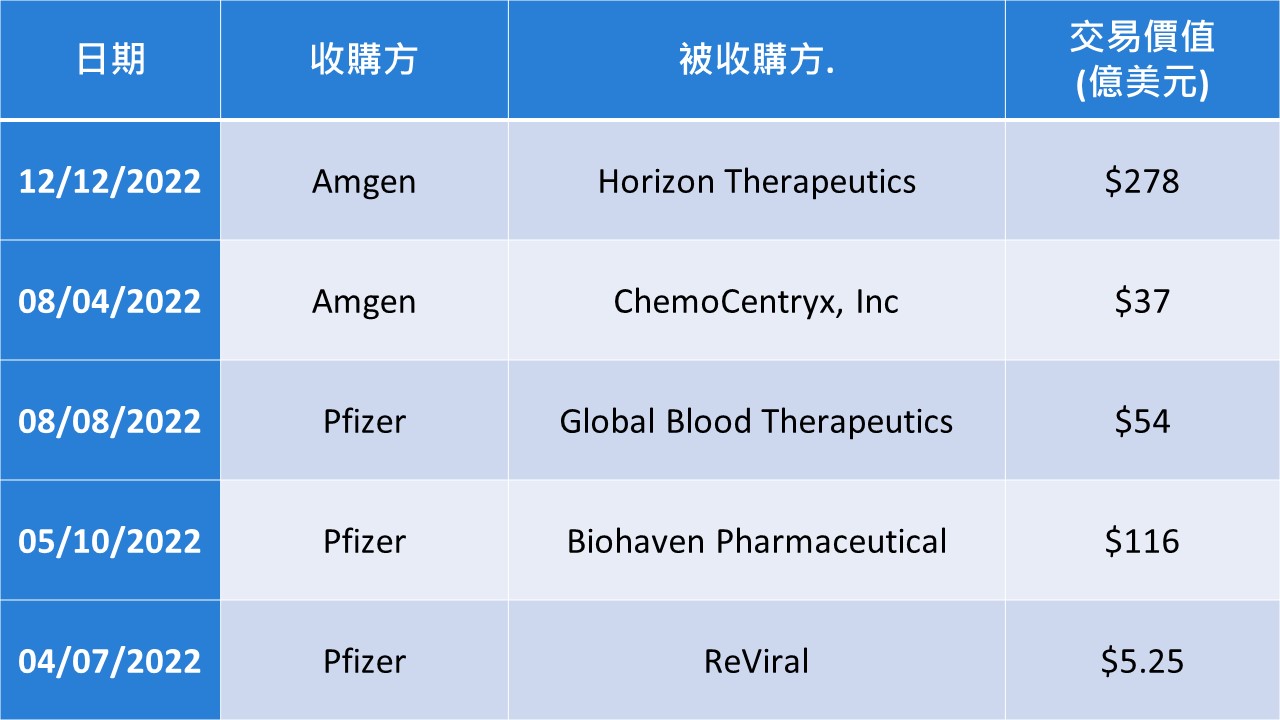
Source: Ji-Pu Industrial Trend Research Institute
Related news link: News
Amgen acquires orphan drug maker Horizon Therapeutics for US$26.4 billion|MaxOffice|MaxOffice.com
[/fusion_text][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container]